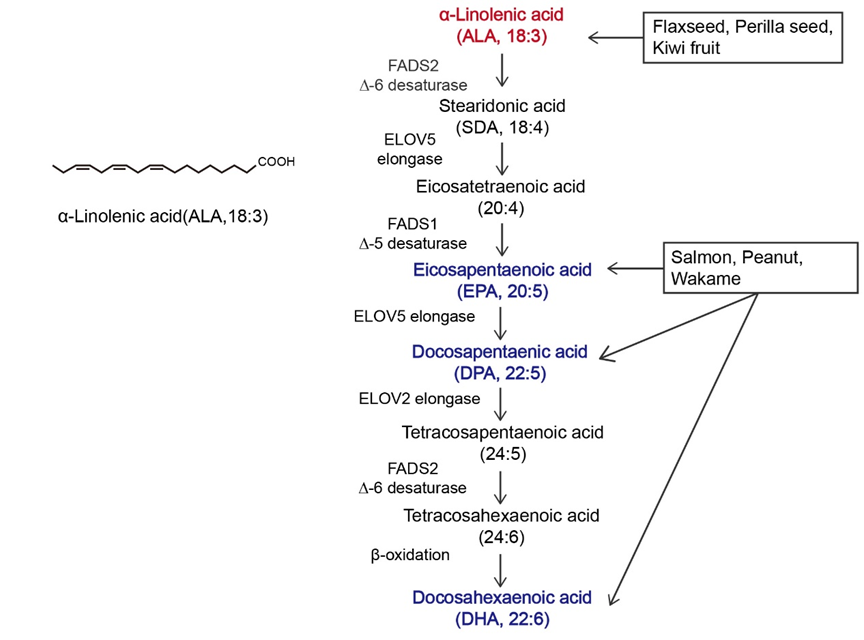
Table 1. The mechanism of the antitumor effects of ALA.
| Cancer |
Effect |
Effector Molecules |
Change in Ex-Pression |
PCa
(prostate cancer) [52] |
anti-inflammatory effect |
PG/LTs |
downregulation |
BC
(breast cancer) [38][39] |
anti-inflammatory effect/inhibition of tumor metastasis |
COX2/PGE2/Twist 1 |
downregulation |
HCC
(hepatocellular carcinoma) [40][41] |
inhibition of proliferation |
Farnesoid X receptor |
upregulation |
CRC
(colorectal cancer) [42][43] |
induction of apoptosis |
caspase 3 |
downregulation |
PCA
(pancreatic cancer) [44] |
anti-inflammatory effect |
IL-1β/IL-6 |
downregulation |
2. Inhibition of Proliferation
Cell proliferation plays a key role in life. Normal cell proliferation is critical for organismal growth, development, tissue repair, and metabolism. However, the abnormal expression of cancer-related genes in cells caused by various factors can lead to uncontrolled cell proliferation, which is an important part of cancer development.
Esophageal tumors can lead to dysphagia and strongly affect patient quality of life. In clinical practice, small and localized tumors are often surgically removed, but there is no way to perform surgery on larger or non-localized tumors. In addition, the resistance of esophageal cancer to chemotherapy has made the need for new therapies more urgent. Hyun-Seuk Moon’s team
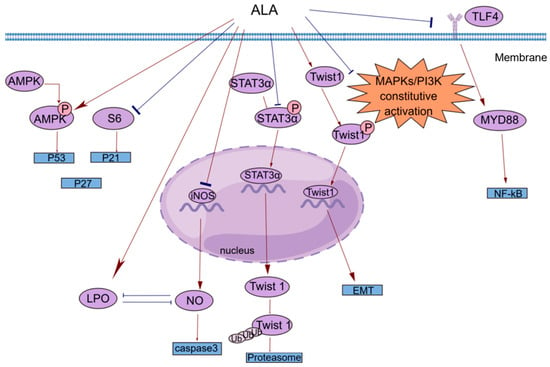
[53] reported that dietary ALA with or without oleic acid (OA) could inhibit the proliferation of the esophageal cancer cell lines OE19 and OE33 by regulating the AMPK/S6 axis to treat esophageal tumors. OA and ALA promoted the expression of tumor suppressor genes, such as p53, p21, and p27, by activating AMPK and/or decreasing the phosphorylation of S6. This provides a new idea for cancer treatment involving the consumption of ALA-containing foods for therapeutic purposes. Studies have shown that ALA alone or in combination with other drugs can inhibit cell proliferation in a variety of ways. Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a nuclear receptor that regulates lipid homeostasis. As natural ligands of PPAR-γ, fatty acids can inhibit the growth of cancer cells by activating PPAR-γ
[54]. Lijun Yang et al.
[55] reported that ALA dose-dependently inhibited the proliferation of renal cell carcinoma (RCC) cells by significantly increasing PPAR-γ activity and gene expression and significantly inhibiting cyclooxygenase-2 (COX-2). COX-2 is an inducible enzyme involved in inflammation. Moreover, when ALA was combined with the PPAR-γ agonist rosiglitazone and the COX-2 inhibitor N-(3-pyridyl) indomethacinamide, its inhibitory effect on the proliferation of the human RCC cell line OS-RC-2 was further increased. ALA inhibited the transformation of cervical cancer cells by reducing the expression of the human papillomavirus oncoproteins E6 and E7, restoring the expression of the tumor suppressor proteins p53 and Rb, and reducing the expression of phosphorylated ERK1/2 and p38. Thus, cell proliferation was inhibited
[56]. Consequently, it is possible and promising to use ALA in clinical practice to treat associated tumors by preventing tumor cell proliferation.
3. Induction of Apoptosis
Apoptosis is regulated by genes and is a type of programmed cell death. During embryonic development, certain cell populations undergo apoptosis to eliminate certain cells and complete organogenesis. However, mutagenesis by external factors can cause normal cells to become cancerous. The carcinogenesis of normal cells requires uncontrolled cell proliferation, the dysfunction of cell apoptosis, and the dysregulation of apoptotic regulators. In general, cancerous cells escape apoptosis by upregulating antiapoptotic factors and downregulating proapoptotic factors
[57].
Most studies investigating the antiapoptotic effects of n-3 PUFAs have focused on major substances such as EPA and DHA, and little research has been conducted on ALA
[58][59][60]. However, the functions of these substances are different. For example, one prospective study separated DHA, EPA, and ALA and found that ALA was the only n-3 PUFA that significantly reduced BC risk
[17]. Interestingly, this phenomenon of local generalization is not uncommon. Caspases are a class of cysteine proteases that can mediate apoptosis. The apoptotic effect of ALA is closely related to its ability to increase lipid peroxidation
[61]. An increase in lipid peroxides may increase the generation of free radicals, and reactive oxygen species (ROS) can directly activate mitochondrial permeability transition, leading to the loss of mitochondrial membrane potential. This results in cytochrome c (cyt c) release and caspase pathway activation. ALA reduced the mRNA expression of inducible nitric oxide synthase (iNOS) to reduce intracellular levels of NO, which can inhibit lipid peroxidation by scavenging free radicals from lipid peroxidation. ALA also inhibited iNOS-induced NO production in a peroxidation-dependent manner, further activating caspase 3 to induce apoptosis
[61]. In addition, studies have shown that ALA can promote apoptosis in a variety of ways, such as by upregulating the expression of the proapoptotic gene Bax, downregulating the expression of the antiapoptotic gene Bcl-2, stabilizing hypoxia-inducible factor-1α (HIF-1α) and downregulating fatty acid synthase (FASN) to promote mitochondrial apoptosis
[62][63]. This opens up multiple possibilities for the clinical use of ALA.
4. Anti-Inflammatory Response
The inflammatory response is a double-edged sword. When the normal balance of the body is disrupted, immune activity is increased and typically manifests as inflammation. However, the existence of inflammation itself and the changes in the microenvironment caused by inflammation cause certain pathological symptoms. Cancer patients are prone to secondary inflammatory diseases, and cancer may also develop in response to inflammation
[64][65]. For example, patients with inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease have an increased risk of CRC and a higher mortality rate than patients with sporadic CRC
[66]. The main reason may be the recurrent chronic inflammatory response, which continuously damages the intestinal mucosa. The mucosa is in a state of long-term repair accompanied by intestinal microbial heterotopia and atypical hyperplasia, which can eventually lead to cancer
[67]. ALA has been shown to exert powerful anti-inflammatory effects
[68], and different epidemiological studies have shown that ALA is inversely correlated with plasma levels of inflammatory factors, including C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-1β (IL-1β), interferon γ (IFN-γ), tumor necrosis factor-α (TNF-α), and E-selectin
[25][26]. The potential anti-inflammatory mechanisms of ALA are diverse and include reducing the level of arachidonic acid (AA) in the blood and downregulating COX-2
[69].
A common clinical phenomenon in alcoholic hepatitis patients is the leakage of bacterial endotoxins through the damaged intestinal barrier into the portal vein. The endotoxins then bind to lipopolysaccharide-binding protein (LBP), triggering the TLR4/MyD88/NF-κB inflammatory cascade in the liver. ALA can inhibit the lipopolysaccharide (LPS)-induced inflammatory response by blocking this cascade
[70]. N-6 polyunsaturated fatty acids, such as linolenic acid (LA), can be metabolized to AA, which is the precursor of many potent proinflammatory mediators, including prostaglandins (PGs) and leukotrienes (LTs)
[71]. ALA intake can reduce blood levels of AA because ALA competes with LA for the enzyme delta-6-desaturase. Moreover, ALA is the preferred substrate of this enzyme. Increasing ALA intake can limit the conversion of LA to AA, thereby reducing the biosynthesis of proinflammatory eicosanoid acid and further exerting anticancer effects
[52][72]. COX-2 enzymes are involved in the synthesis of proinflammatory prostaglandins, and one possible mechanism by which ALA inhibits cancer is that it inhibits inflammation through the downregulation of COX-2
[73]. NF-κB plays an important role in the inflammatory and immune responses. COX-2 is a downstream target of NF-κB activation. ALA suppresses tumors by reducing the expression of NF-κB and its target genes in tumor cells
[56].
5. Inhibition of Tumor Metastasis
Tumors can spread in vivo to local normal tissues, to nearby lymph nodes, tissues, and organs, or to distant tissues through fluid transport, which is a feature that has made them a leading cause of cancer-related death
[74]. Cancer metastasis can be divided into the following steps: tumor growth in situ, angiogenesis, epithelial–mesenchymal transition (EMT), invasion, intravasation, survival in the blood circulation, extravasation, dormancy, and metastatic tumor growth
[75].
In vivo and in vitro experiments have shown that ALA inhibits metastasis in various cancers to varying degrees. Marianela Vara-Messler et al.
[76] fed BALB/c mice a chia seed oil diet rich in ALA and corn oil rich in LA as a control and found that ALA could significantly reduce the incidence of BC and the number of metastatic lesions in mice. Mechanistically, ALA can alter signaling in BC cells by altering the cell membrane structure, which increases fatty acid unsaturation and exerts anticancer effects. In vitro experiments were performed to explore the mechanisms by which ALA inhibits cancer metastasis, including the EMT and tumor angiogenesis. Twist1 is required to initiate EMT and promote tumor metastasis and is regulated by signal transducer and activator of transcription 3α (STAT3α) and mitogen-activated protein kinases (MAPKs). Shih-Chung Wang et al.
[39] studied the effect of ALA on Twist1 and Twist1-mediated TNBC cell migration and reported that ALA could reduce the mRNA expression of Twist1, reduce the accumulation of STAT3α in the nucleus, and reduce the protein and phosphorylation levels of Twist1. ALA promoted Twist1 degradation, thereby eliminating EMT and inhibiting Twist1-mediated migration in TNBC cells. One of the hallmark features of EMT is the loss of epithelial integrity due to the degradation of adherens junctions, which maintain epithelial cell contacts. A major driver of this degradation is proteolytic digestion by matrix metalloproteinases (MMPs). In tumors, MMP2 and MMP9 are involved in connective tissue degradation, tumor-induced angiogenesis, and cell migration. Another mechanism by which ALA inhibits tumor metastasis is by reducing vascular endothelial growth factor (VEGF), MMP-2 and MMP-9 protein expression
[56]. As an important vasodilator, NO can stimulate VEGF production and participate in every step of VEGF-mediated tumor angiogenesis. NO can be generated by the enzyme iNOS in vivo. ALA not only reduces NO levels by reducing the mRNA expression of iNOS but also increases lipid peroxidation. The accumulation of peroxides increases the generation of free radicals; thus, the inactivation and reduction of NO decrease tumor angiogenesis and inhibit tumor metastasis
[56][61].
6. Antioxidant Effect
Oxidative stress (OS) refers to the breakdown of the balance between ROS production and elimination in the body and is mainly characterized by the excessive production of highly reactive molecules such as ROS and reactive nitrogen species (RNS). OS is closely related to the occurrence and development of tumors
[77][78]. Rashmi Deshpande et al.
[61]’s study revealed that ALA could inhibit cancer by stimulating ROS production to induce apoptosis. However, this study was performed in a relatively simple in vitro experimental environment, and there are more complex and diverse mechanisms in vivo that can counteract this effect. Leslie Couedelo et al.
[79] showed that ALA intake induced vitamin E depletion. Since vitamin E is a potent antioxidant, it can be used to capture the free radicals produced without producing OS. In addition, Jin Hyang Song and Teruo Miyazawa reported that excessive incorporation of n-3 PUFAs into cell membranes can have adverse effects by enhancing membrane sensitivity to lipid peroxidation (LPO) and inducing OS, but these studies were based on a high-fat diet
[80]. In conclusion, when ALA is added to the diet, its effect on the body as a whole should be considered, and the amount of intake should also be considered.
Flaxseed oil (FO) is rich in ALA and is often used as a dietary supplement to treat cancer and improve health. Jyoti Sharma et al.
[81] examined mice with skin cancer induced by 7, 12-dimethylbenzo-[a] thane (DMBA) combined with croton oil and showed that FO scavenged free radicals by increasing the levels of enzymatic and nonenzymatic antioxidants, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione (GSH), in the skin and liver. SOD, CAT, and GPx are important antioxidant enzymes that work in concert to prevent excessive levels of intracellular ROS. The main role of SOD is to accelerate the dismutation of superoxide anions to hydrogen peroxide, after which CAT and GPx convert the resulting hydrogen peroxide into harmless substances
[82]. The free radical scavenging effect of GSH is mediated by the active sulfhydryl-SH groups in its structure, which are easily dehydrogenated through oxidation. In addition, GSH can bind to GPx and requires different secondary enzymes and cofactors, including nicotinamide–adenine dinucleotide phosphate (NADPH), to exert its effects
[81]. NADPH oxidase, which is the major source of ROS in the vasculature, is composed of a catalytic subunit and a regulatory cytosolic subunit. Hao Han
[83] examined atherosclerosis and showed that FO could inhibit NADPH oxidase by reducing the mRNA and protein expression of the NADPH oxidase catalytic subunit, thereby regulating cytoplasmic subunit expression, reducing malondialdehyde levels and increasing GSH levels to exert antioxidant effects. ALA-rich FO is consumed mainly as a cooking oil, and there is undoubtedly a close association between this nutrient and the gut microbiota. Xiaoyan Sun et al.
[84] used an FO diet to study the antioxidant capacity of colon epithelial cells in aged rats, and the results showed that the antioxidant levels of aged rats increased significantly after FO administration. Mechanistically, the intake of FO may have changed some intestinal bacteria. OS can play a role in the entire process of tumor development, and ALA intake can slow the toxic effects on the body when administered at different stages of tumor development.