
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 1354 | 2024-03-13 14:03:09 | | | |
| 2 | Peter Tang | Meta information modification | 1354 | 2024-03-14 03:40:26 | | |
Video Upload Options
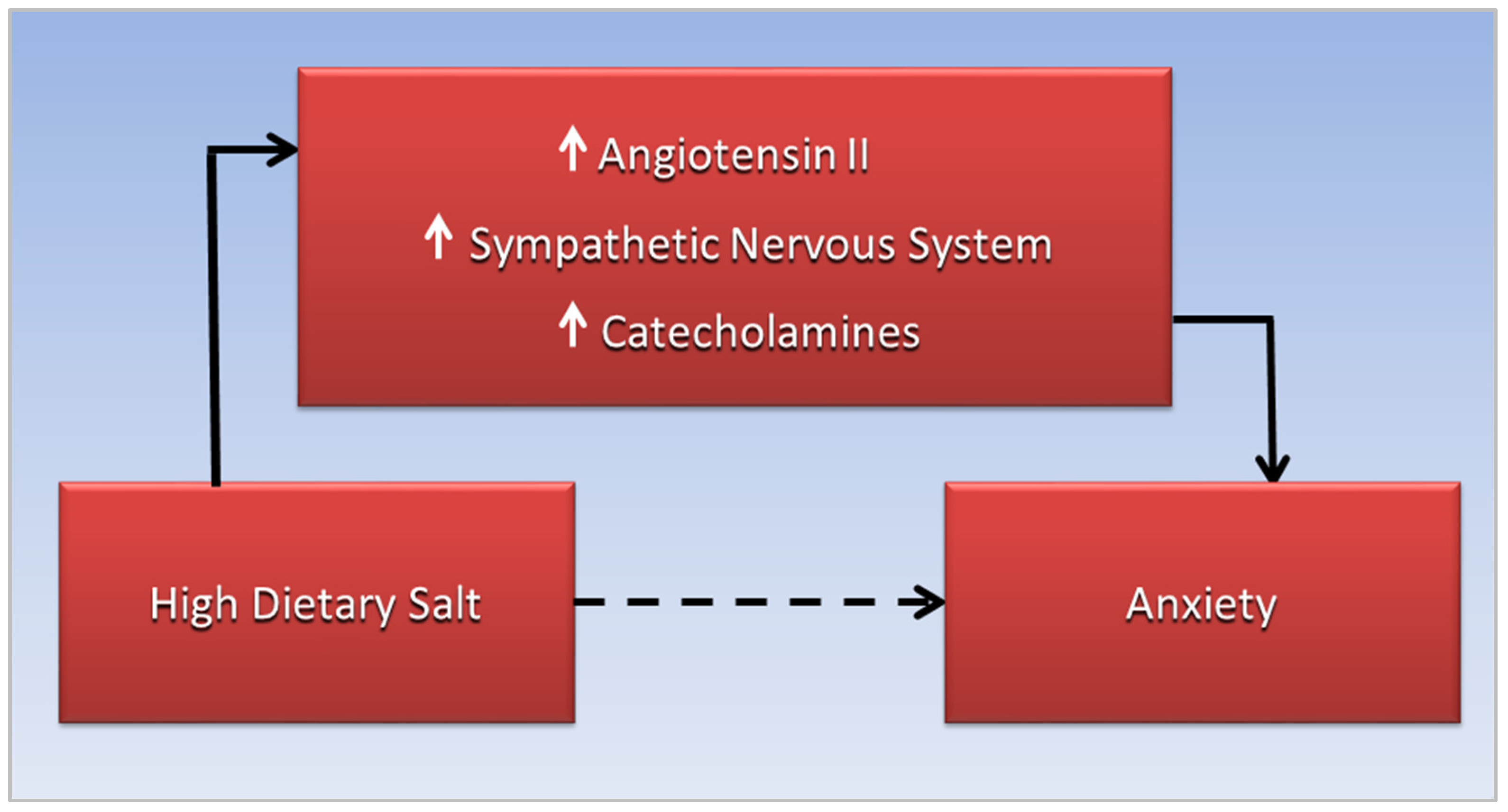
Increased anxiety in these conditions may be linked to a high-salt diet through stimulation of the sympathetic nervous system, which increases blood pressure while releasing catecholamines, causing a “fight or flight” response. A rostral shift of fluid overload from the lower to the upper body occurs in obstructive sleep apnea associated with COVID-19 and cardiovascular disease, and may be related to sodium and fluid retention triggered by hypertonic dehydration. Chronic activation of the renin-angiotensin-aldosterone system responds to salt-induced dehydration by increasing reabsorption of sodium and fluid, potentially exacerbating fluid overload. Anxiety may also be related to angiotensin II that stimulates the sympathetic nervous system to release catecholamines.
1. Introduction
2. Anxiety, CVD, and Sodium Toxicity
3. OSA, Hypertension, and the Renin-Angiotensin-Aldosterone System
4. OSA, Anxiety, and Angiotensin II
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292.
- Wu, C.Y.; Hu, H.Y.; Chou, Y.J.; Huang, N.; Chou, Y.C.; Li, C.P. High Blood Pressure and All-Cause and Cardiovascular Disease Mortalities in Community-Dwelling Older Adults. Medicine 2015, 94, e2160.
- Hall, J.E.; Guyton, A.C.; Coleman, T.G.; Mizelle, H.L.; Woods, L.L. Regulation of arterial pressure: Role of pressure natriuresis and diuresis. Fed. Proc. 1986, 45, 2897–2903.
- Who.Int. Salt Reduction. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 30 August 2022).
- Choi, H.Y.; Park, H.C.; Ha, S.K. Salt Sensitivity and Hypertension: A Paradigm Shift from Kidney Malfunction to Vascular Endothelial Dysfunction. Electrolytes Blood Press. 2015, 13, 7–16.
- Grillo, A.; Salvi, L.; Coruzzi, P.; Salvi, P.; Parati, G. Sodium intake and hypertension. Nutrients 2019, 11, 1970.
- Rust, P.; Ekmekcioglu, C. Impact of Salt Intake on the Pathogenesis and Treatment of Hypertension. In Hypertension: From Basic Research to Clinical Practice; Islam, M.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–84.
- Luzardo, L.; Noboa, O.; Boggia, J. Mechanisms of Salt-Sensitive Hypertension. Curr. Hypertens. Rev. 2015, 11, 14–21.
- Janszky, I.; Ahnve, S.; Lundberg, I.; Hemmingsson, T. Early-Onset Depression, Anxiety, and Risk of Subsequent Coronary Heart Disease: 37-Year Follow-Up of 49,321 Young Swedish Men. J. Am. Coll. Cardiol. 2010, 56, 31–37.
- Roest, A.M.; Martens, E.J.; de Jonge, P.; Denollet, J. Anxiety and risk of incident coronary heart disease: A meta-analysis. J. Am. Coll. Cardiol. 2010, 56, 38–46.
- Batelaan, N.M.; Seldenrijk, A.; Bot, M.; van Balkom, A.J.; Penninx, B.W. Anxiety and new onset of cardiovascular disease: Critical review and meta-analysis. Br. J. Psychiatry 2016, 208, 223–231.
- Reiner, I.C.; Tibubos, A.N.; Werner, A.M.; Ernst, M.; Brähler, E.; Wiltink, J.; Michal, M.; Schulz, A.; Wild, P.S.; Münzel, T.; et al. The association of chronic anxiousness with cardiovascular disease and mortality in the community: Results from the Gutenberg Health Study. Sci. Rep. 2020, 10, 12436.
- Dimsdale, J.E. What does heart disease have to do with anxiety? J. Am. Coll. Cardiol. 2010, 56, 47–48.
- Goddard, A.W.; Ball, S.G.; Martinez, J.; Robinson, M.J.; Yang, C.R.; Russell, J.M.; Shekhar, A. Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress. Anxiety 2010, 27, 339–350.
- Ralph, A.F.; Grenier, C.; Costello, H.M.; Stewart, K.; Ivy, J.R.; Dhaun, N.; Bailey, M.A. Activation of the Sympathetic Nervous System Promotes Blood Pressure Salt-Sensitivity in C57BL6/J Mice. Hypertension 2021, 77, 158–168.
- Pan, Y.; Cai, W.; Cheng, Q.; Dong, W.; An, T.; Yan, J. Association between anxiety and hypertension: A systematic review and meta-analysis of epidemiological studies. Neuropsychiatr. Dis. Treat. 2015, 11, 1121–1130.
- Peskind, E.R.; Radant, A.; Dobie, D.J.; Hughes, J.; Wilkinson, C.W.; Sikkema, C.; Veith, R.C.; Dorsa, D.M.; Raskind, M.A. Hypertonic saline infusion increases plasma norepinephrine concentrations in normal men. Psychoneuroendocrinology 1993, 18, 103–113.
- Molosh, A.I.; Johnson, P.L.; Fitz, S.D.; Dimicco, J.A.; Herman, J.P.; Shekhar, A. Changes in central sodium and not osmolarity or lactate induce panic-like responses in a model of panic disorder. Neuropsychopharmacology 2010, 35, 1333–1347.
- Arnold, E. Anxiety DSM-5 Diagnostic Criteria and Treatment Overview. Available online: https://pro.psycom.net/assessment-diagnosis-adherence/anxiety (accessed on 30 August 2022).
- Yi, B.; Titze, J.; Rykova, M.; Feuerecker, M.; Vassilieva, G.; Nichiporuk, I.; Schelling, G.; Morukov, B.; Choukèr, A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: A longitudinal study. Transl. Res. 2015, 166, 103–110.
- da Silva, B.C.; Kasai, T.; Coelho, F.M.; Zatz, R.; Elias, R.M. Fluid Redistribution in Sleep Apnea: Therapeutic Implications in Edematous States. Front. Med. 2017, 4, 256.
- Bangash, A.; Wajid, F.; Poolacherla, R.; Mim, F.K.; Rutkofsky, I.H. Obstructive Sleep Apnea and Hypertension: A Review of the Relationship and Pathogenic Association. Cureus 2020, 12, e8241.
- Dominguez, A.; Muppidi, V.; Gupta, S. Hyperaldosteronism. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499983/ (accessed on 1 October 2022).
- Coble, J.P.; Grobe, J.L.; Johnson, A.K.; Sigmund, C.D. Mechanisms of brain renin angiotensin system-induced drinking and blood pressure: Importance of the subfornical organ. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R238–R249.
- Tiarks, G. Hypertonic dehydration: What Is It, Causes, Treatment, and More. Available online: https://www.osmosis.org/answers/hypertonic-dehydration (accessed on 1 September 2022).
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382.
- Augustine, R.; Abhilash, S.; Nayeem, A.; Salam, S.A.; Augustine, P.; Dan, P.; Maureira, P.; Mraiche, F.; Gentile, C.; Hansbro, P.M.; et al. Increased complications of COVID-19 in people with cardiovascular disease: Role of the renin–angiotensin-aldosterone system (RAAS) dysregulation. Chem. Biol. Interact. 2022, 351, 109738.
- Dudenbostel, T.; Calhoun, D.A. Resistant hypertension, obstructive sleep apnoea and aldosterone. J. Hum. Hypertens. 2012, 26, 281–287.
- Fereidoun, H.; Pouria, H. Effect of excessive salt consumption on night’s sleep. Pak J. physiol. 2014, 10, 6–9.
- Pimenta, E.; Stowasser, M.; Gordon, R.D.; Harding, S.M.; Batlouni, M.; Zhang, B.; Oparil, S.; Calhoun, D.A. Increased dietary sodium is related to severity of obstructive sleep apnea in patients with resistant hypertension and hyperaldosteronism. Chest 2013, 143, 978–983.
- Fiori, C.Z.; Martinez, D.; Montanari, C.C.; Lopez, P.; Camargo, R.; Sezerá, L.; Gonçalves, S.C.; Fuchs, F.D. Diuretic or sodium-restricted diet for obstructive sleep apnea—A randomized trial. Sleep 2018, 41, zsy016.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Sodium and Potassium; Oria, M., Harrison, M., Stallings, V.A., Eds.; National Academies Press (US): Washington, DC, USA, 2019.
- NHLBI. Implementing Recommendations for Dietary Salt Reduction: Where Are We? Where Are We Going? How Do We Get There?: A Summary of an NHLBI Workshop; National Institutes of Health, National Heart, Lung, and Blood Institute: Bethesda, MD, USA, 1996.
- Kasai, T.; Arcand, J.; Allard, J.P.; Mak, S.; Azevedo, E.R.; Newton, G.E.; Bradley, T.D. Relationship between sodium intake and sleep apnea in patients with heart failure. J. Am. Coll. Cardiol. 2011, 58, 1970–1974.
- Kim, J.Y.; Ko, I.; Kim, D.K. Association of Obstructive Sleep Apnea With the Risk of Affective Disorders. JAMA Otolaryngol. –Head Neck Surg. 2019, 145, 1020–1026.
- Rezaeitalab, F.; Moharrari, F.; Saberi, S.; Asadpour, H.; Rezaeetalab, F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J. Res. Med. Sci. 2014, 19, 205–210.
- Cox, R.C.; Olatunji, B.O. Sleep in the anxiety-related disorders: A meta-analysis of subjective and objective research. Sleep Med. Rev. 2020, 51, 101282.
- Akberzie, W.; Hesselbacher, S.; Aiyer, I.; Surani, S.; Surani, Z.S. The Prevalence of Anxiety and Depression Symptoms in Obstructive Sleep Apnea. Cureus 2020, 12, e11203.
- Duan, X.; Zheng, M.; Zhao, W.; Huang, J.; Lao, L.; Li, H.; Lu, J.; Chen, W.; Liu, X.; Deng, H. Associations of Depression, Anxiety, and Life Events With the Risk of Obstructive Sleep Apnea Evaluated by Berlin Questionnaire. Front. Med. 2022, 9, 799792.
- Daabis, R.; Gharraf, H. Predictors of anxiety and depression in patients with obstructive sleep apnea. Egypt. J. Chest Dis. Tuberc. 2012, 61, 171–177.
- Garbarino, S.; Bardwell, W.A.; Guglielmi, O.; Chiorri, C.; Bonanni, E.; Magnavita, N. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav. Sleep Med. 2020, 18, 35–57.
- Batzikosta, A.; Antoniadou, M.; Tiga, P.; Nena, E.; Xanthoudaki, M.; Voulgaris, A.; Sotiropoulou, R.; Kouratzi, M.; Froudarakis, M.; Steiropoulos, P. AB011. Assessment of anxiety and depressive symptoms in obstructive sleep apnea patients. Ann. Transl. Med. 2016, 4, 11.
- Wong, J.L.; Martinez, F.; Aguila, A.P.; Pal, A.; Aysola, R.S.; Henderson, L.A.; Macey, P.M. Stress in obstructive sleep apnea. Sci. Rep. 2021, 11, 12631.
- Sharafkhaneh, A.; Giray, N.; Richardson, P.; Young, T.; Hirshkowitz, M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep 2005, 28, 1405–1411.
- Mansukhani, M.P.; Kara, T.; Caples, S.M.; Somers, V.K. Chemoreflexes, sleep apnea, and sympathetic dysregulation. Curr. Hypertens. Rep. 2014, 16, 476.
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257.
- Wang, Y.; Seto, S.W.; Golledge, J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail. Rev. 2014, 19, 187–198.
- Dendorfer, A.; Thornagel, A.; Raasch, W.; Grisk, O.; Tempel, K.; Dominiak, P. Angiotensin II induces catecholamine release by direct ganglionic excitation. Hypertension 2002, 40, 348–354.
- Hakim, F.; Gozal, D.; Kheirandish-Gozal, L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front. Neurol. 2012, 3, 7.
- Sica, E.; De Bernardi, F.; Nosetti, L.; Martini, S.; Cosentino, M.; Castelnuovo, P.; Marino, F. Catecholamines and children obstructive sleep apnea: A systematic review. Sleep Med. 2021, 87, 227–232.




