Hypertension, or high blood pressure, is a leading risk factor for cardiovascular disease (CVD) [
1], and hypertension’s impact on mortality in aging adults is predicted to increase over the next few decades [
2]. Arterial pressure is regulated by lowering fluid retention and hypervolemia from high dietary salt intake [
3]. The World Health Organization recommendations for salt reduction advise that “less than 5 g per day for adults helps to reduce blood pressure and risk of cardiovascular disease, stroke and coronary heart attack”; yet, “most people consume too much salt—on average 9–12 g per day, or around twice the recommended maximum level of intake” [
4]. Other blood pressure mechanisms affected by excessive salt intake include vascular endothelial dysfunction [
5], “changes in the structure and function of large elastic arteries”, as well as “modification in sympathetic activity, and in the autonomic neuronal modulation of the cardiovascular system” [
6]. Blood pressure is reduced considerably in hypertensive patients when dietary salt intake is reduced, and smaller reductions occur in people with normal blood pressure [
7]. Some individuals have higher sensitivity to the effects of dietary salt than other people, but “there is no consensus for a definition of salt sensitivity and the precise mechanisms that explain their association are not yet fully understood” [
8].
2. Anxiety, CVD, and Sodium Toxicity
Diagnosis of anxiety according to criteria of the International Classification of Diseases-8 (ICD-8) was associated with an increased risk of subsequent coronary heart disease events in 49,321 Swedish men [
61]. A 2010 meta-analysis of 20 studies found a higher incidence of coronary heart disease and cardiac mortality associated with anxiety, prompting the researchers to suggest that anxiety was an independent risk factor for cardiac morbidity and mortality [
62]. More recently, a 2016 meta-analysis of 37 studies found that anxiety was associated with a 52% increased risk of CVD prevalence [
63], and new onset CVD was associated with anxiousness in a 2020 study of a German population [
64]. Nevertheless, the underlying mechanisms causatively linking anxiety with CVD are unknown. In an editorial in the Journal of the American College of Cardiology, Dimsdale [
65] speculated that leading pathophysiological mediators in the causative pathway between anxiety and heart disease include “sympathetic nervous system activity and various inflammatory markers”. Dimsdale further noted the need to scrutinize potential underlying risk factors that are common to anxiety and CVD.
The adrenal catecholamines epinephrine and norepinephrine of the sympathetic nervous system increase the “fight-or-flight” stress response, and dysregulation of this response under conditions of chronic stress can contribute to anxiety [
66]. A high-salt diet in a model of salt-sensitive mice was found to stimulate an overactive response of the sympathetic nervous system, which was associated with increased blood pressure and increased levels of adrenal epinephrine production [
67]. Additionally, a systematic review and meta-analysis of epidemiological studies found that hypertension is associated with anxiety [
68]. Hypertonic saline infusion also increased activity of the sympathetic nervous system and raised plasma norepinephrine levels in normal men [
69]. Similarly, hypertonic saline induced panic attacks in an animal model of panic disorder [
70]. This evidence suggests an anxiogenic link with high dietary salt intake, which hypothetically may satisfy criteria for toxin exposure in substance/medication-induced anxiety disorder, listed in the Diagnostic and Statistics Manuel-5 (DSM-5) [
71]. Furthermore, an inflammatory response induced by high salt intake in healthy humans increases interleukin-6 (IL-6) and IL-23 pro-inflammatory cytokines, while reducing anti-inflammatory cytokine IL-10 [
72].
3. OSA, Hypertension, and the Renin-Angiotensin-Aldosterone System
A nocturnal rostral shift that redistributes fluid overload from the lower body towards the head occurs in OSA [
73], exacerbating obstruction in the upper airways and increasing blood pressure in patients with hypertension [
74]. Secondary hyperaldosteronism, often present in OSA, occurs from excessive activation of the renin-angiotensin-aldosterone system (RAAS), which can be due to edematous disorders [
75]. RAAS activation increases salt and fluid reabsorption in the kidneys which “is important for restoring homeostasis after dehydration”, and thirst responses to intracellular dehydration are mediated by angiotensin II type 2 receptors (AT
2R) [
76]. Importantly, infusion of hypertonic sodium chloride (hypernatremia) causes intracellular hypertonic dehydration [
77], suggesting that excessive ingestion of sodium chloride and hypertonic dehydration could trigger RAAS activation and possibly chronic overcompensation as reabsorbed salt and fluid levels contribute to hypervolemia. Chronic RAAS activation causing tissue remodeling and dysfunction occurs in congestive heart failure, systemic hypertension, and chronic kidney disease [
78]. Dysregulated RAAS response is also implicated in COVID-19 complications in patients with CVD [
79]. The RAAS response related to edema and hypervolemia from high dietary salt intake could also explain excessive aldosterone levels associated with parapharyngeal edema and upper airway resistance in severe OSA [
80].
Excessive salt consumed by 20 student volunteers in a study of OSA found that “the normal pattern of sleep was disturbed and the depth of sleep was decreased” [
81]. A 2013 study found that OSA was prevalent in 77.3% of patients with resistant hypertension and hyperaldosteronism, and an increase in OSA severity was associated with high dietary salt intake [
82]. The researchers hypothesized that high dietary salt intake was a causative factor in the study findings, and suggested that “interventional studies that use dietary salt restriction as a treatment strategy for OSA in patients with resistant hypertension and hyperaldosteronism are needed to test this hypothesis”.
Subsequently, results of a randomized trial published in 2018 found only minor reductions in OSA severity after one week of testing the use of diuretics and reduced dietary sodium [
83]. However, the sodium-restricted group in the study ingested a daily maximum intake of 3 g sodium, which is higher than the U.S. Dietary Reference Intake (DRI) of 2300 mg sodium advised to reduce chronic disease risk in adults, twice as high as the DRI of 1500 mg sodium considered adequate for adults [
84], and six times higher than essential sodium requirements of 500 mg recommended by the U.S. National Heart, Lung, and Blood Institute [
85]. Furthermore, a case–control study of sleep apnea in heart failure patients found that patients with sleep apnea had a mean daily sodium intake of 3000 mg compared to patients without sleep apnea with a mean daily sodium intake of 1900 mg [
86]. More research is needed to test interventions with lower daily levels of sodium intake (500 mg–1500 mg) for OSA prevention.
4. OSA, Anxiety, and Angiotensin II
Anxiety is associated with OSA and sleep disorders [
87,
88,
89,
90,
91,
92,
93,
94,
95,
96], although causative relationships are not clear and require more investigations. Additionally, sympathetic nervous system response is increased and parasympathetic response is decreased in OSA, the opposite effect of normal sleep, and increased variability of heart rate and blood pressure often extends into daytime wakefulness with normal breathing [
97], suggesting causative factors involving the RAAS response.
Angiotensin II (AngII) of the RAAS response, derived from angiotensin I through action of angiotensin-I converting enzyme, increases blood pressure and retention of sodium and fluids, but as humans adopted a salt diet, the protective effects of the RAAS response turned into “a negative factor” [
98]. “Plasma angiotensin II is increased in humans and animals with chronic heart failure” [
99]. Additionally, AngII is “known to facilitate catecholamine release from peripheral sympathetic neurons by enhancing depolarization-dependent exocytosis”, contributing to vasoconstriction and sodium retention [
100]. Of relevance, elevated levels of catecholamines are present in the urine and serum of patients with OSA, including children with OSA [
101,
102]. This evidence suggests that increased interaction of AngII with the sympathetic nervous system and increased release of catecholamines forms a potential mechanism that mediates the association of high dietary salt with anxiety, proposed in
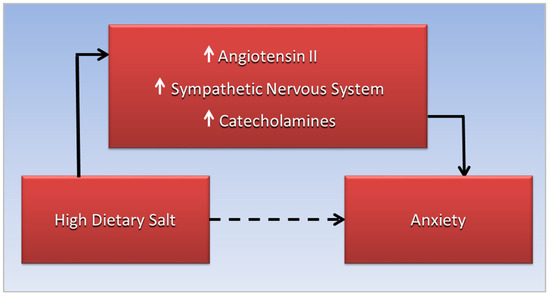
Figure 1. More research is needed to explore this anxiogenic mechanism.
Figure 1. The association of high dietary salt and anxiety, the dotted line, is mediated by increased interaction of angiotensin II with the sympathetic nervous system, leading to increased catecholamine release.