
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Keith M Kendrick | -- | 2621 | 2024-03-13 08:46:02 | | | |
| 2 | Fanny Huang | Meta information modification | 2621 | 2024-03-18 02:34:15 | | |
Video Upload Options
The neuropeptide oxytocin is synthesized by cells in the hypothalamic paraventricular and supraoptic nuclei and transported to the posterior pituitary for release into the blood, where it is most well-known for acting on smooth muscle to stimulate uterine contractions during labor and milk-ejection from the breast. The role of the hypothalamic neuropeptide oxytocin in influencing the brain and behavior has been the subject of widespread research due, most notably, to its reported involvement in promoting social cognition and motivation, reducing anxiety, and relieving pain. It is also increasingly being considered as an important therapeutic intervention in a variety of disorders with social dysfunction as a symptom. There is increasing evidence that many of its functional effects can be peripherally mediated via increasing its concentration in the blood. This has opened up an oromucosal administration route as an alternative, which is beneficial since the oral consumption of peptides is problematic due to their rapid breakdown in the acidic environment of the gastrointestinal system.
1. Introduction
2. Oromucosal Administration of Peptides
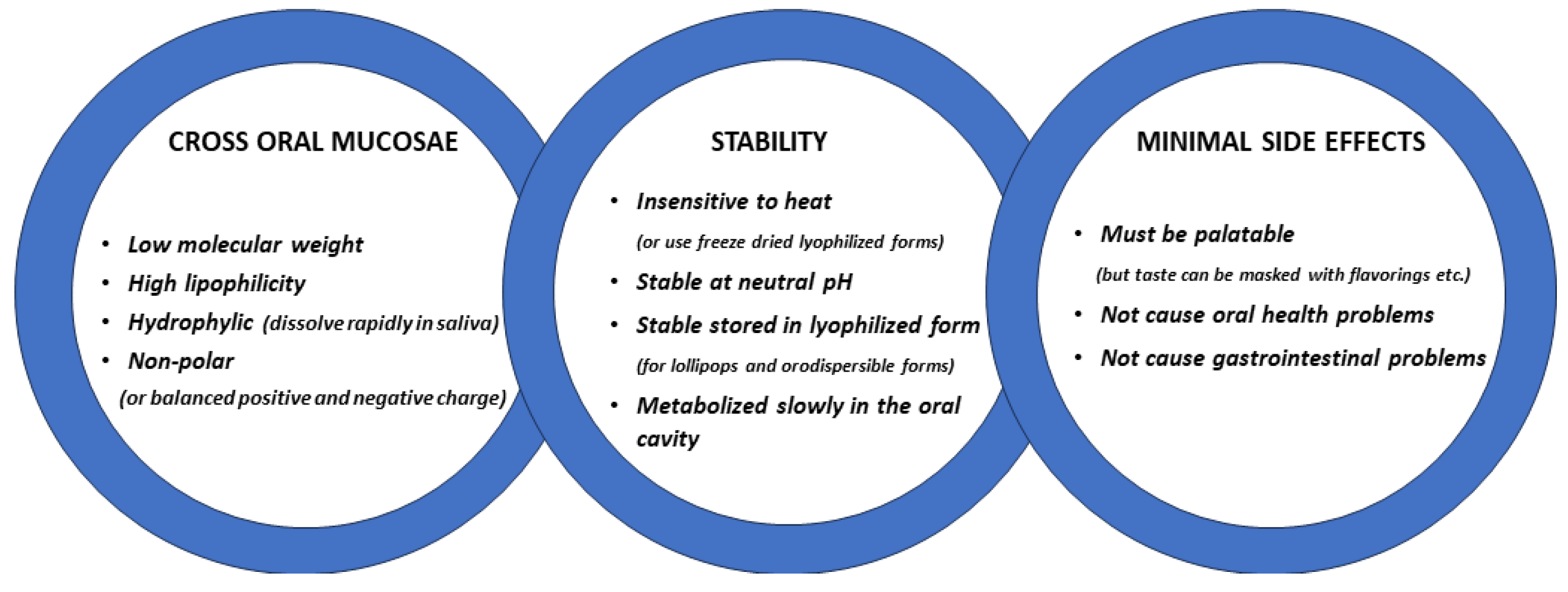
Development of Oromucosal Oxytocin Administration Using Lingual Spray and Medicated Lollipop ‘Oxipop’ Methodologies

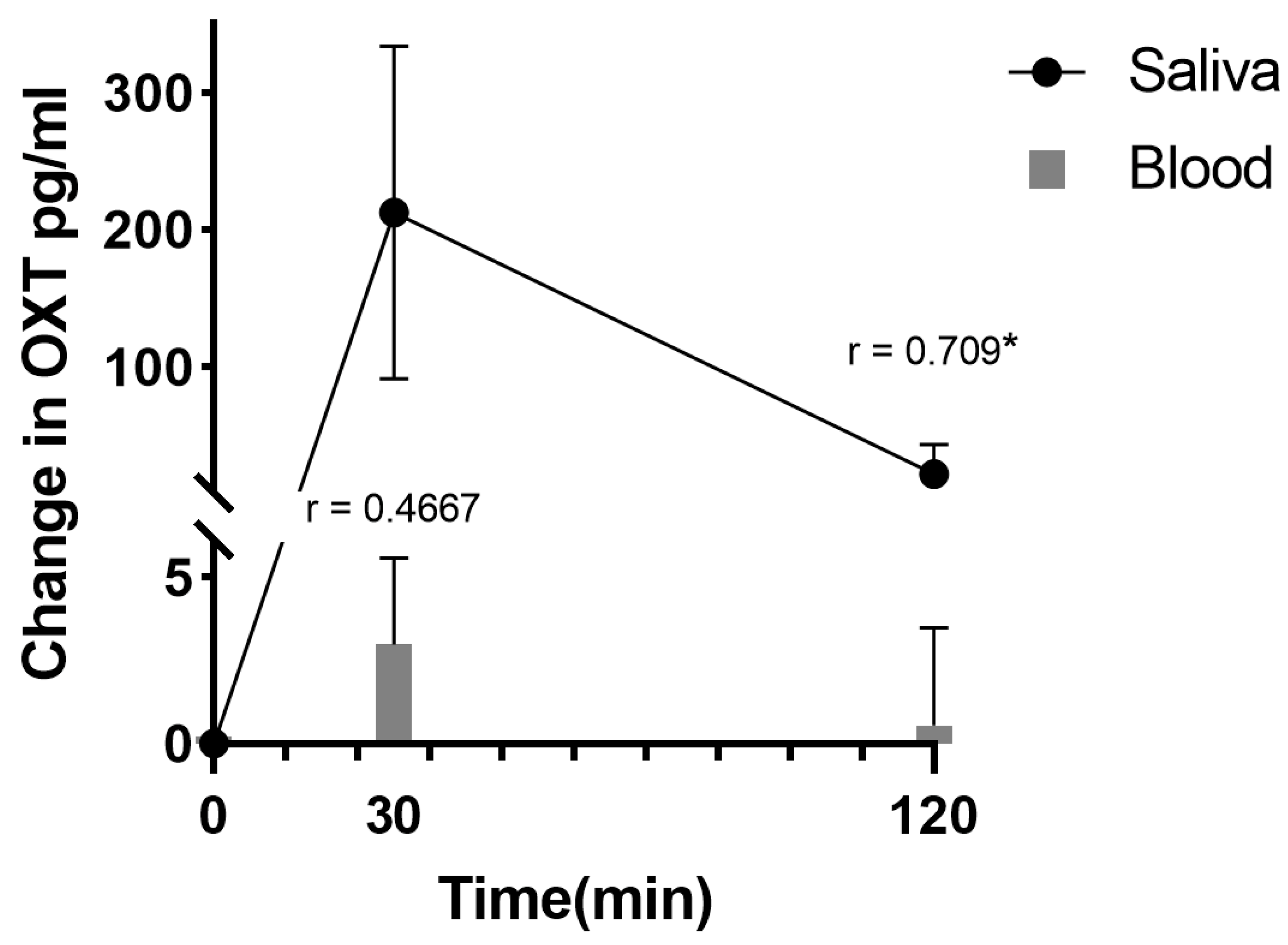
3. Neural and Behavioral Effects of Oromucosal Administration of Oxytocin
References
- Hermesch, A.C.; Kernberg, A.S.; Layoun, V.R.; Caughey, A.B. Oxytocin: Physiology, pharmacology, and clinical application for labor management. Am. J. Obstet. Gynecol. 2023; in press.
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683.
- Kendrick, K.M.; Guastella, A.J.; Becker, B. Overview of human oxytocin research. Curr. Top. Behav. Neurosci. 2017, 35, 321–348.
- Yao, S.; Kendrick, K.M. Effects of Intranasal Administration of Oxytocin and Vasopressin on Social Cognition and Potential Routes and Mechanisms of Action. Pharmaceutics 2022, 14, 323.
- Le, J.; Zhang, L.; Zhao, W.; Zhu, S.; Lan, C.; Kou, J.; Zhang, Q.; Zhang, Y.; Li, Q.; Chen, Z.; et al. Infrequent Intranasal Oxytocin Followed by Positive Social Interaction Improves Symptoms in Autistic Children: A Pilot Randomized Clinical Trial. Psychother. Psychosom. 2022, 91, 335–347.
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124.
- Yamasue, H.; Kojima, M.; Kuwabara, H.; Kuroda, M.; Matsumoto, K.; Kanai, C.; Inada, N.; Owada, K.; Ochi, K.; Ono, N.; et al. Effect of a novel nasal oxytocin spray with enhanced bioavailability on autism: A randomized trial. Brain 2022, 145, 490–499.
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231.
- De Cagna, F.; Fusar-Poli, L.; Damiani, S.; Rocchetti, M.; Giovanna, G.; Mori, A.; Politi, P.; Brondino, N. The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2019, 17, 1–11.
- Bharadwaj, V.N.; Tzabazis, A.Z.; Klukinov, M.; Manering, N.A.; Yeomans, D.C. Intranasal Administration for Pain: Oxytocin and Other Polypeptides. Pharmaceutics 2021, 13, 1088.
- Van Eyk, A.D.; Vand der Bijl, P.; Moll, L.M. Physicochemical characteristics of molecules and their diffusion across human vaginal mucosa. Eur. J. Inflamm. 2008, 2, 65–71.
- Prego, C.; Garcia, M.; Torres, D.; Alonso, M.J. Transmucosal macromolecular drug delivery. J. Control. Release 2005, 101, 151–162.
- Frokjaer, S.; Otzen, D.E. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005, 4, 298–306.
- Rathbone, M.J.; Drummond, B.K.; Tucker, I.G. The oral cavity as a site for systemic drug delivery. Adv. Drug Deliv. Rev. 1994, 13, 1–22.
- Bastos, F.; Pinto, A.C.; Nunes, A.; Simões, S. Oromucosal products—Market landscape and innovative technologies: A review. J. Control. Release 2022, 348, 305–320.
- Mehta, A.C. Buccal and oral drugs: Induction of labour. Acta. Chir. Hung. 1986, 27, 157–163.
- Westergaard, J.G.; Lange, A.P.; Pedersen, G.T.; Secher, N.J. Oral oxytocics for induction of labor. A randomized study of prostaglandin E2 tablets and demoxytocin resoriblets. Acta. Obstet. Gynecol. Scand. 1983, 62, 103–110.
- Gleeson, J.P.; Fein, K.C.; Whitehead, K.A. Oral delivery of peptide therapeutics in infants: Challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 173, 112–124.
- Sharman, A.; Low, J. Vasopressin and its role in critical care. Cont. Ed. Anaesth. Crit. Care Pain 2008, 8, 134–137.26.
- van Kerrebroeck, P.; Nørgaard, J.P. Desmopressin for the treatment of primary nocturnal enuresis. Pediatr. Health 2009, 3, 311–327.
- Schiele, J.T.; Quinzler, R.; Klimm, H.-D.; Pruszydlo, M.G.; Haefeli, W.E. Difficulties swallowing solid oral dosage forms in a general practice population: Prevalence, causes, and relationship to dosage forms. Eur. J. Clin. Pharmacol. 2013, 69, 937–948.
- FDA. 2020. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/ferring-us-issues-voluntary-nationwide-recall-ddavpr-nasal-spray-10-mcg01ml-desmopressin-acetate (accessed on 8 May 2020).
- Kottke, D.; Burckhardt, B.B.; Knaab, T.C.; Breitkreutz, J.; Fischer, B. Development and evaluation of a composite dosage form containing desmopressin acetate for buccal administration. Int. J. Pharm. X 2021, 9, 100082.
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Exp. Op. Drug Deliv. 2011, 8, 299–316.
- Chen, Y.; Zou, H.; Hou, X.; Lan, C.; Wang, J.; Qing, Y.; Chen, W.; Yao, S.; Kendrick, K.M. Oxytocin administration enhances pleasantness and neural responses to gentle stroking but not moderate pressure social touch by increasing peripheral concentrations. Elife 2023, 12, e85847.
- Kou, J.; Lan, C.; Zhang, Y.; Wang, Q.; Zhou, F.; Zhao, Z.; Montag, C.; Yao, S.; Becker, B.; Kendrick, K.M. In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing. Transl. Psychiatry 2021, 11, 94.
- Zhuang, Q.; Zheng, X.; Yao, S.; Zhao, W.; Becker, B.; Xu, X.; Kendrick, K.M. Oral Administration of Oxytocin, Like Intranasal Administration, Decreases Top-Down Social Attention. Int. J. Neuropsychopharmacol. 2022, 25, 912–923.
- Martins, D.A.; Mazibuko, N.; Zelaya, F.; Vasilakopoulou, S.; Loveridge, J.; Oates, A.; Maltezos, S.; Mehta, M.; Wastling, S.; Howard, M.; et al. Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans. Nat. Commun. 2020, 11, 1160.
- Pawar, P.G.; Darekar, A.B.; Saudagar, R.B. Medicated chocolate and lollipops: A novel drug delivery system for pediatric patients. Pharma. Sci. Monitor. 2018, 9, 677–696.
- Shetty, S.; Kamath, K.; Shabaraya, R.; Miranda, F.C. Design and development of medicated lollipop containing albendazole. Am. J. Pharmatech. Res. 2019, 9, 275–285.
- Tangso, K.J.; Ho, Q.P.; Boyd, B.J. Confectionery-based dose forms. Curr. Drug Deliv. 2015, 12, 56–62.
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237.
- Xu, D.; Li, Q.; Zhuang, Q.; Zhang, Y.; Yao, S.; Zhao, W.; Kendrick, K.M. Oro-mucosal administration of oxytocin using medicated lollipops alters social attention, similar to intranasal and lingual routes: Implications for therapeutic use. Front. Neurosci. 2022, 16, 1022101.
- Daughters, K.; Manstead, A.S.R.; Hubble, K.; Rees, A.; Thapar, A.; van Goozen, S.H.M. Salivary Oxytocin Concentrations in Males following Intranasal Administration of Oxytocin: A Double-Blind, CrossOver Study. PLoS ONE 2015, 10, e0145104.
- van Ijzendoorn, M.H.; Bhandari, R.; van der Veen, R.; Grewen, K.M.; Bakermans-Kranenburg, M.J. Elevated Salivary Levels of Oxytocin Persist More than 7 h after Intranasal Administration. Front. Neurosci. 2012, 6, 174.
- Weisman, O.; Zagoory-Sharon, O.; Feldman, R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology 2012, 37, 1582–1586.
- Weisman, O.; Schneiderman, I.; Zagoory-Sharon, O.; Feldman, R. Salivary vasopressin increases following intranasal oxytocin administration. Peptides 2013, 40, 99–103.
- Martins, D.; Gabay, A.S.; Mehta, M.; Paloyelis, Y. Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. Elife 2020, 9, e62456.
- Striepens, N.; Kendrick, K.M.; Hanking, V.; Landgraf, R.; Wüllner, U.; Maier, W.; Hurlemann, R. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci. Rep. 2013, 3, 3440.
- Kou, J.; Zhang, Y.; Zhou, F.; Sindermann, C.; Montag, C.; Becker, B.; Kendrick, K.M. A randomized trial shows dose-frequency and genotype may determine the therapeutic efficacy of intranasal oxytocin. Psychol. Med. 2022, 52, 1959–1968.
- Spengler, F.B.; Schultz, J.; Scheele, D.; Essel, M.; Maier, W.; Heinrichs, M.; Hurlemann, R. Kinetics and Dose Dependency of Intranasal Oxytocin Effects on Amygdala Reactivity. Biol. Psychiatry 2017, 82, 885–894.
- Lieberz, J.; Scheele, D.; Spengler, F.B.; Matheisen, T.; Schneider, L.; Stoffel-Wagner, B.; Kinfe, T.M.; Hurlemann, R. Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology 2020, 45, 1134–1140.
- Lan, C.; Chen, Y.; Zhang, Y.; Kou, J.; Huang, L.; Xu, T.; Yang, X.; Xu, D.; Yang, W.; Kendrick, K.M.; et al. Oral Oxytocin Facilitates Responses to Emotional Faces in Reward and Emotional-Processing Networks in Females. Neuroendocrinology 2023, 113, 957–970.




