Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mathis Brette Mortensen | -- | 3104 | 2024-01-05 13:47:53 | | | |
| 2 | Camila Xu | Meta information modification | 3104 | 2024-01-09 02:02:04 | | | | |
| 3 | Camila Xu | -8 word(s) | 3096 | 2024-01-09 02:24:08 | | | | |
| 4 | Mathis Brette Mortensen | Meta information modification | 3096 | 2024-01-09 09:00:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brette, M.M.; Holm, A.H.; Drozdov, A.D.; Christiansen, J.D.C. Molecular Weight of Polyamides. Encyclopedia. Available online: https://encyclopedia.pub/entry/53485 (accessed on 10 March 2026).
Brette MM, Holm AH, Drozdov AD, Christiansen JDC. Molecular Weight of Polyamides. Encyclopedia. Available at: https://encyclopedia.pub/entry/53485. Accessed March 10, 2026.
Brette, Mathis Mortensen, Allan Hjarbæk Holm, Aleksey D. Drozdov, Jesper De Claville Christiansen. "Molecular Weight of Polyamides" Encyclopedia, https://encyclopedia.pub/entry/53485 (accessed March 10, 2026).
Brette, M.M., Holm, A.H., Drozdov, A.D., & Christiansen, J.D.C. (2024, January 05). Molecular Weight of Polyamides. In Encyclopedia. https://encyclopedia.pub/entry/53485
Brette, Mathis Mortensen, et al. "Molecular Weight of Polyamides." Encyclopedia. Web. 05 January, 2024.
Copy Citation
Polyamides (PAs) undergo local environmental degradation, leading to a decline in their mechanical properties over time. PAs can experience various forms of degradation, such as thermal degradation, oxidation, hydrothermal oxidation, UV oxidation, and hydrolysis. In order to better comprehend the degradation process of PAs, it is crucial to understand each of these degradation mechanisms individually.
hydrolysis
polyamide
aging
degradation
crystallization
embrittlement
1. Introduction
Polyamides (PAs) are thermoplastic polymers thriving as household and engineering materials because of their high elasticity, mechanical strength, high melting point, chemical resistance to oil, and barrier properties against liquids and gases. These properties make them adequate not only as carpets, stockings, and clothing as fibers but also gears, pipelines, and automotive components as plastics. In society, they are commonly referred to as nylon [1][2][3][4]. In their applications, PAs are exposed to chemical aging, which negatively impacts their mechanical properties in the long term. When in contact with water or humidity for a long period of time, PAs can undergo hydrolysis. This causes a cut within the polymer chains, leading to a decrease in molecular weight over time [5][6].
While the degradation mechanism of PAs in water is seemingly straightforward from a chemical point of a view, when diving deeper, it can be found that a complex coupling of mechanisms occurs. Water reacts with the amide group and induces chain scission. This is the reverse polymerization reaction from the condensation of the acid and amine monomers. The presence of oxygen both in the air and dissolved in the aqueous solution is of great importance. This oxygen oxidizes the polymer, which not only further degrades it, but also introduces a whole new set of different chemical degradation pathways. Thermal oxidation starts with the abstraction of a hydrogen at the aliphatic carbon chain by oxygen radicals. The carbon atoms adjacent to the amide groups turn into radicals from the homolytic cleavage of their hydrogen [5][7][8][9][10]. This further leads the material to experience yellowing, discoloration, cross-linking, embrittlement, and so on. The thermal oxidation of PAs has been investigated in many articles over the years [9][11][12][13][14][15][16][17][18][19].
2. Molecular Weight
2.1. Polyamide 6
PA6 has many intrinsic valuable properties such as high mechanical strength, high chemical resistance, low friction coefficient, and transparency. The polymer is not unaffected by water degradation due to the polarity of its amide bonds, which ultimately impacts its molecular weight overtime [20]. The molecular weight measurements presented here are all originating from gel permeation chromatography (GPC) measurements. Arhant et al. [21] immersed a 5 mm thick commercial PA6 containing a 60 wt% carbon fiber (C) reinforcement in water for up to 90 days with temperatures ranging from 100 ∘C to 140 ∘C. The changes in the number average molecular weight (Mn) of the polymer over time are shown in Figure 1. Starting at 26.2 kg/mol, Mn quickly drops within the first days and slowly reaches an equilibrium over time. For an aging temperature of 120 ∘C, Mn decreased from 26.2 kg/mol to 15.5 kg/mol within the first 10 days of aging, until reaching an equilibrium at around 11.0 kg/mol after 28 days. Increasing the aging temperature has two separate effects on Mn. Higher temperatures accelerate the decrease in Mn during the early-stage hydrolysis as well as the lowering of Mn at equilibrium. At 140 ∘C, the equilibrium was reached after only 9 days with a Mn of 8 kg/mol.
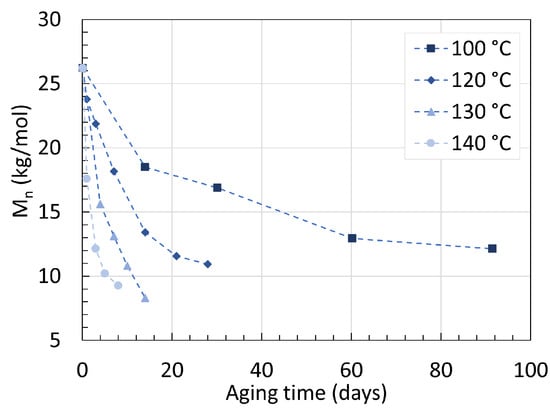
Figure 1. Changes in Mn of PA6/C over time at 100, 120, 130, and 140 ∘C when immersed in water. Data from [21].
On top of the Mn, the polydispersity index (PDI) or the distribution of molecular mass in a polymer was recorded as well. The PDI remained constant at 2.3 all throughout the process, indicating that pure hydrolysis is a random process occurring at all locations of the chains.
To investigate the coupling effect of hydrolysis and oxidation, Deshoulles et al. [22] aged their 0.25 mm thick neat PA6 material in dry hot air, deoxygenated water, and oxygenated water at 80 ∘C for long periods of time. Their GPC data are presented in Figure 2. The starting Mn was 52.3 kg/mol for all three degradation mechanisms. In deoxygenated water, Mn reached 32.5 kg/mol after 700 days of aging. In dry hot air, Mn reached 13.8 kg/mol after 630 days, making thermal oxidation a faster degradation process than pure hydrolysis at 80 ∘C. And finally, when immersed in oxygenated hot water, Mn fell drastically down to 13.5 kg/mol after merely 28 days, 80 times faster than pure hydrolysis. This underlines the importance of carefully removing oxygen from the aging chamber both in the solution and above it. The PDIs recorded for all three mechanisms are all dissimilar to one another. Similar to the previous data from Arhant et al. [21] as well as an earlier work from Deshoulles et al. [23], the PDI for pure hydrolysis at 80 ∘C remained at 2.3 constantly. For thermal oxidation, the PDI rose from 2.3 to 2.9 after the 630 days of aging. The authors issued this phenomenon to the cross-linking taking place at the last stage of oxidation. The PDI rose to even higher values when both mechanisms were combined. A PDI of nearly 4.5 after the 28 days of aging was reported, indicating that the chain scission process occurs in a less random manner compared to pure hydrolysis.
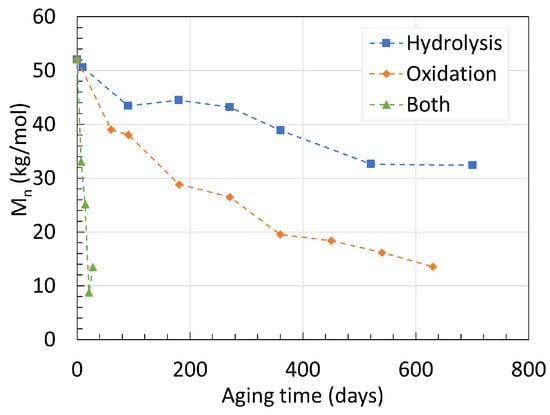
Figure 2. Changes in Mn of neat PA6 over time and degradation mechanisms at 80 ∘C. Data from [22].
To decouple both mechanisms further, a sample of neat PA6 was aged in dry hot air until molecular weight equilibrium, then placed in deoxygenated hot water. It is noted that the equilibrium reached when aged under oxidation and hydrolysis is different, as oxidation includes radical chemistry which leads not only to the formation of amines and carboxylic acids, but also cross-linking, bridging, and more. The results are shown in Figure 3. Both mechanisms were taking place at 100 ∘C to accelerate the procedure. The Mn decreased further even after the pre-oxidative treatment. This phenomenon is linked to the formation of imides during oxidation before chain scission, enhancing the likelihood of hydrolysis occurring from these chemical groups. This has been proven through Fourier-transform infrared (FTIR) spectroscopy.
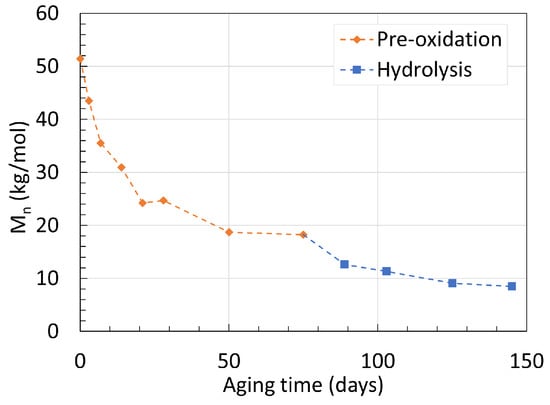
Figure 3. Changes in Mn of neat PA6 over time with a pre-oxidative stage at 100 ∘C. Data from [22].
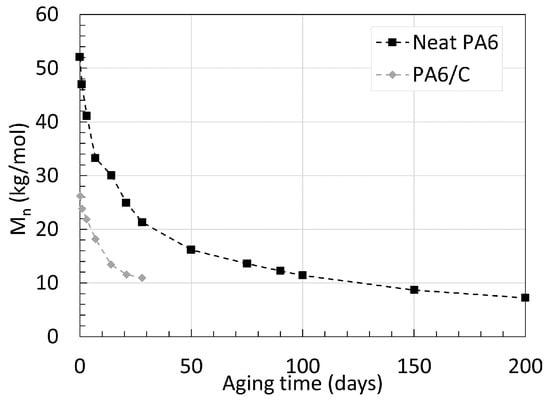
When comparing the decrease in Mn of PA6/C and neat PA6, one can notice the significantly slower drop in Mn of the neat PA6 over time. At 80 ∘C, the neat PA6 obtained a Mn 32.4 kg/mol after 700 days of aging, compared to the PA6/C that reached a Mn of 12.1 kg/mol after only 90 days of aging at 100 ∘C; both reached equilibrium. The fibers were described by Arhant et al. [21] as being indifferent to both the effect of water and temperatures as high as 120 ∘C. Hence, the contrast may originate solely from the small difference in aging temperature, pointing out that an exponentially higher degradation rate occurs with aging temperatures higher than 80 ∘C for PA6. This indication is further supported by looking at an earlier work from Deshoulles et al. [23], where, again, neat PA6 with a thickness of 0.25 mm was aged in deoxygenated water, but at 120 ∘C until equilibrium. Both trends are shown in Figure 4. The Mn of both PA6s decreases to a similar value. Notably, a higher starting Mn also seems to result in a faster degradation rate. In the case of glass fibers, the presence of reinforcement has been associated to a lower water absorption property due to the simple fact that less PA is present in the network when reinforced [24]. If the two PA6 were to start at similar Mn, it is likely that the reinforced PA would degrade slower.
Another earlier work from Deshoulles et al. [25] aimed at determining a parameter able to track the embrittlement or, i.e., the ductile to brittle transition of PA6. Neat PA6 without additives was aged in deoxygenated water and dry hot air separated once again for up to almost 2 years with temperatures ranging from 80 ∘C to 140 ∘C. The samples had a thickness of 250 m and 75 m, respectively. The PDI recorded from aging through both pure hydrolysis and pure thermal oxidation remained equal to 2.5 ± 0.5. This high deviation created a large uncertainty, preventing a direct comparison to other PDI values from other sources. The changes in Mn are shown in Figure 5 for both pure hydrolysis and Figure 6 for pure thermal oxidation.
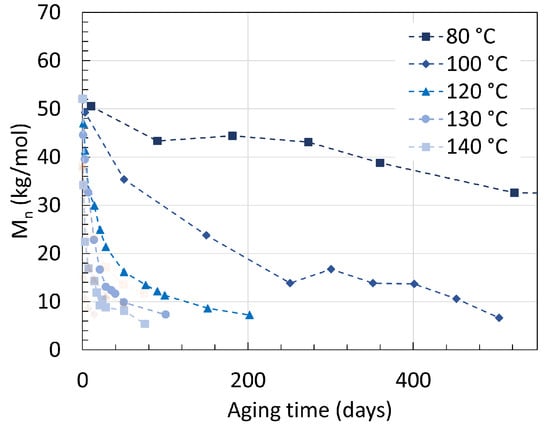
Figure 5. Changes in Mn for neat PA6 without additives in deoxygenated water with varying temperatures. The dotted lines are here for illustrations and does not represent a mathematical fitting. Each reaction temperature has a shape and symbol respectively. The see-through plot in the background corresponds to the data for oxidation. Data from [25].
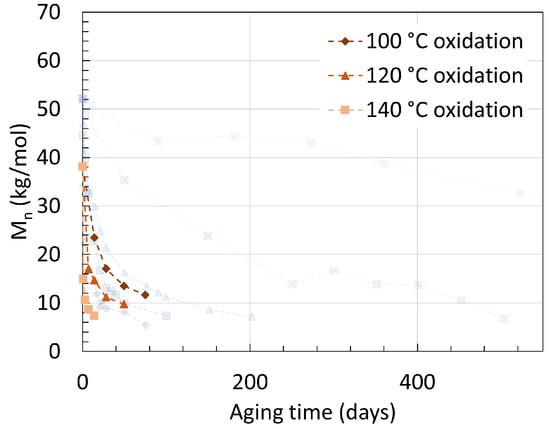
Figure 6. Changes in Mn for neat PA6 without additives in dry hot air with varying temperatures. The dotted lines are here for illustrations and does not represent a mathematical fitting. Each reaction temperature has a shape and symbol respectively. The see-through plot in the background corresponds to the data for hydrolysis. Data from [25].
Degrading PA6 in dry air led to a faster drop in Mn than degrading it in deoxygenated water at high temperatures. For pure hydrolysis, Mn reached 32.6 kg/mol after 520 days of aging at 80 ∘C, and this value decreased exponentially to 6.7 kg/mol when rising the temperature from 80 ∘C to 100 ∘C for about the same aging time. This is in correlation with the previous statement that a significant effect is to be expected at the 80 ∘C to 100 ∘C temperature range. Increasing the temperature further led to a faster drop rate and a slight decrease in Mn at equilibrium.
2.2. Polyamide 11
In addition to PA6, knowing the degradation behavior of PA11 in deoxygenated water has also been of great interest. This comes from one of its main applications as internal pressure sheath in offshore underwater flexible pipes where the concentration of oxygen is scarce [26]. PA11 harbors a high chemical resistance, with a working temperature ranging from −40 to 130 ∘C. [27]. PA11 also undergoes hydrolysis like PA6 or PA66. The main difference lies behind PA11’s water affinity worsened by its longer carbon chain. Meyer et al. [28] aged an unplasticized, laboratory-made 3 mm thick neat PA11 in deoxygenated water at temperatures ranging from 90 ∘C to 135 ∘C with varying starting average molecular weight (Mw). The Mw is another way of describing the molecular weight of polymers and is always greater than Mn. Factoring the Mn with the PDI provides Mw. The variations of Mw over time at 90 ∘C and 135 ∘C are displayed in Figure 7.
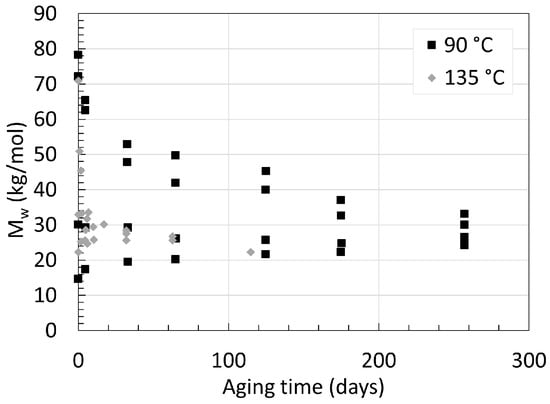
Figure 7. Changes in Mw for neat PA11 at 90 ∘C and 135 ∘C for up to 260 days. Data from [28].
Regardless of the starting Mw, all Mw values tend towards an equilibrium. The accelerating effect is more than obvious when looking at the Mw after around 65 days of aging for both 90 ∘C and 135 ∘C with 72 kg/mol as a similar starting Mw. At 90 ∘C, Mw reached about 50 kg/mol, while at 135 ∘C, this value reached 25 kg/mol. One can argue visually that the Mw at equilibrium for 135 ∘C tends towards 25 kg/mol, and for 90 ∘C, this equilibrium appears to be at around 28 kg/mol. This would be in line with the previous statement regarding the effect of temperature on PA6 upon its Mn. Another important information is the fact that all PA11s with a starting Mw below the equilibrium have their Mw rising towards it. This increase in Mw is another sign of the reaction equilibrium between hydrolysis and condensation. Mw below such equilibrium implies a higher concentration of primary amine and carboxylic acid end-groups in contrast to the concentration of amide groups in the polymer. Following the basic concept of the Le Châtelier’s principle, this shift induces an enhanced condensation rate of the end groups. Meyer et al. [28] also looked at the effect of pH by aging the same PA11 in acidic aqueous solution with a pH of 3 and 5 using hydrochloric acid. The lower the pH, the faster the hydrolysis rate, and the lower the Mw. At pH 5, the equilibrium shifted from 25 kg/mol to around 22 kg/mol at 120 ∘C. The effect of lower pH is said to protonate the amine end groups to a cation. This ties up the lone pair of electrons initially present on the nitrogen group, preventing the nucleophilic attack of the amine towards the carboxylic acid. This explains the lower condensation rate and the lower Mw at equilibrium at lower pH.
Similar results were obtained from the work of Hocker et al. [29]. Samples of 0.3 mm thick PA11 plasticized with an unspecified amount of N-butylbenzenesulfonamide (BBSA) were aged with different amounts of graphene oxide (GO) as reinforcement at 100 ∘C and 120 ∘C for up to 4 months. Without any fibers, the Mw reached around 24 kg/mol at equilibrium for both temperatures, and the higher aging temperature caused the expected accelerating effect reported several times previously. The increased aging temperature did not lower the Mw significantly, probably due to the lower impact of hydrolysis on PA11 compared to PA6 and the small temperature difference of merely 10 ∘C. At 100 ∘C, the hydrolysis rate and condensation rate reached 2.8×10−2 and 5.0×10−5 day−1. These rates increased to 12×10−2 and 23×10−5 day−1 for 120 ∘C, according to the authors’ data. Additionally, 1 mg/g of GO in PA11 rose Mw at equilibrium by 40% from the neat PA11 for both temperatures and reduced the hydrolysis rate by 1×10−2 day−1 at 100 ∘C and 7.5×10−2 day−1 at 120 ∘C. However, 5 mg/g resulted in the loss of that property from a poor nano-particle dispersion due to agglomeration of the GOs.
Jacques et al. [30] degraded their 3 mm thick 12 wt% BBSA plasticized samples with additional light and heat stabilizers in deoxygenated water for 450 days with temperature ranging from 90 to 140 ∘C. The viscosity was also tracked using rheology. Only the full graph for the degradation at 140 ∘C was provided from GPC. The Mn at equilibrium was provided for the other aging temperature. For PA11, the PDI recorded during pure hydrolysis was always around 1.9, unlike the 2.3 from PA6. The hydrolysis rate rose from 0.42×10−4 kg · mol−1× day−1 at 90 ∘C to 24.1×10−4 kg · mol−1· day−1 at 140 ∘C. It was found that the rate of condensation was 1000 faster than hydrolysis when Mn or Mw were under equilibrium, and this increased exponentially as temperature decreased below 90 ∘C. The data from PA11 aged at 140 ∘C were obtained from Jacques et al. [30], and the data from Hocker et al. [29] at 100 ∘C, and 120 ∘C are displayed in Figure 8 below. Figure 9 displays Mn at equilibrium recorded from Jacques et al. [30] but converted to Mw for easier comparison using their recorded average PDI of 1.9 at 80 ∘C after aging.
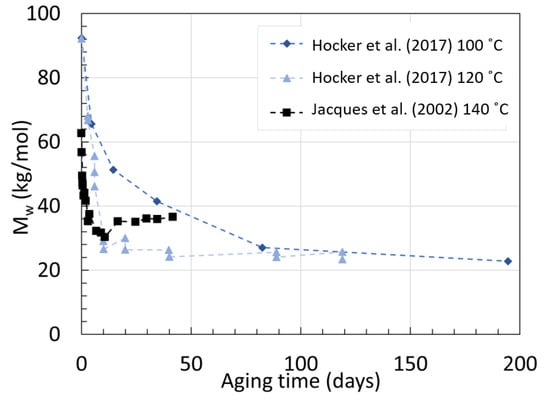
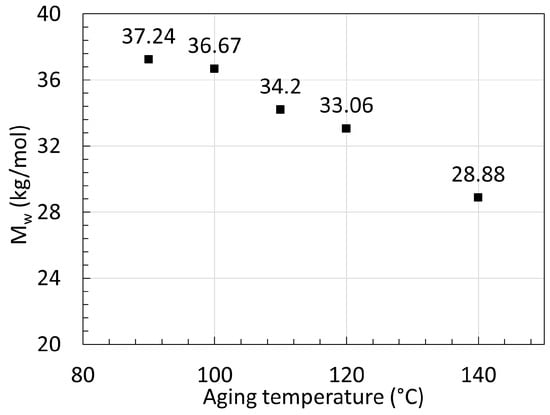
Figure 9. Series of reached Mw at equilibrium from aged PA11 at varying temperatures. Data from [30].
The data from Jacques et al. [30] showed that at 140 ∘C, the Mw decreases fast as expected within the first days, but seems to reach a much higher Mw at equilibrium than the two neat PA11 from Hocker et al. [29] at 100 ∘C and 120 ∘C. This was not discussed by the authors. Both PA11 contain plasticizers and additives but likely not in the same quantity. Jacques et al. [30] mentioned that the polymer contained an unspecified amount of heat and light stabilizer as opposed to the ones from Hocker et al. [29]. The decrease and subsequent increase in Mw may arise from the extraction of such additives.
On top of that, the Mw at equilibrium for 140 ∘C calculated from their tables is lower than the measurements from their graph. The calculated 28.88 kg/mol was found with the assumption that the PDI would remain the same, regardless of the aging temperature. If one takes the direct Mn at equilibrium and the Mw at equilibrium from the graph, a PDI of about 2.3 is calculated. When looking at the data from Meyer et al. [28], this PDI seems to sit at a lower value, closer to 1.6.
Another paper experimenting on the degradation of PA11 in water worth mentioning looked at the changes in Mn and Mw over time and degradation mechanisms. There, Maïza et al. [31] aged their 1.5 mm to 2.0 mm thick PA11 in deoxygenated hot dry air and deoxygenated hot water. The data for both conditions did not differ much and, in them, hydrolysis resulted in a faster reduction in Mw compared to thermal degradation. The authors detected the presence of free radicals formed during the extrusion via FTIR, showcasing the importance of carefully preventing the formation of free radicals from any parts of the experiments, not solely the aging process.
The effect of pH has been briefly mentioned before, but not extensively. When one thinks of reducing the pH of an aging chamber, hydrochloric acid [28][32] or sulfuric acid [33] are often used. An earlier work from Hocker et al. [34] investigated the possibility of using organic acids instead. The authors found that not only do organic acids degrade PAs faster than inorganic acids, but the weaker the organic acid, the faster the degradation. This was attempted with acetic, propanoic, and butanoic acid at a concentration of 1.05 × 10−2 M for all three on their 250 mm to 1 cm laboratory-made thick PA11 sample. For PA11 aged in water, Mw at equilibrium reached 24.4 kg/mol and 26.9 kg/mol for 100 ∘C and 120 ∘C respectively. The higher Mw is likely an exception occurring from the small temperature difference. The rate of hydrolysis, on the contrary, increased from 0.43 ×10−1 to 1.64 ×10−1 from the temperature increase, proving again that the main effect of temperature is acceleration. Another experiment from Hocker et al. [35] was designed to determine the ductile to brittle transition. In this attempt, they also aged two PA11 samples with a thickness of 2 ± 0.5 mm and 0.3 ± 0.02 mm in water, acetic acid, and butanoic acid at 120 ∘C. As the data originate from the same research group and consist of the same experimental setup, the results are combined and depicted in Figure 10. At 120 ∘C, the Mw of neat PA11 at equilibrium reached 26.9, 20.0, 17.0, and 14.5 kg/mol when aged in water, acetic, propanoic, and butanoic acid solutions. Acids have two effects on degradation, a hydrolysis acceleration effect as well as an amine scavenging effect. The solubility of the acid in regard to the PA is the governing factor, not its pH.
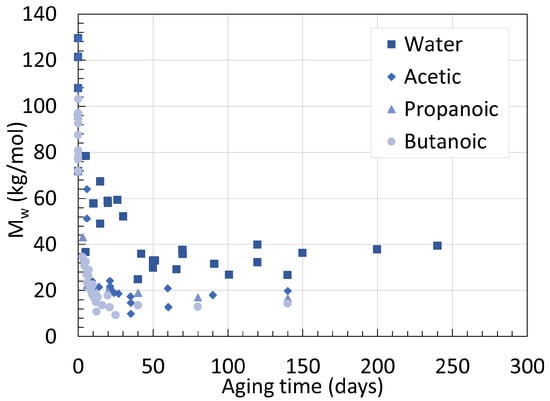
This is well represented in Figure 11, where the variations of Mw over time of plasticized PA11 aged in different acidic conditions are shown.
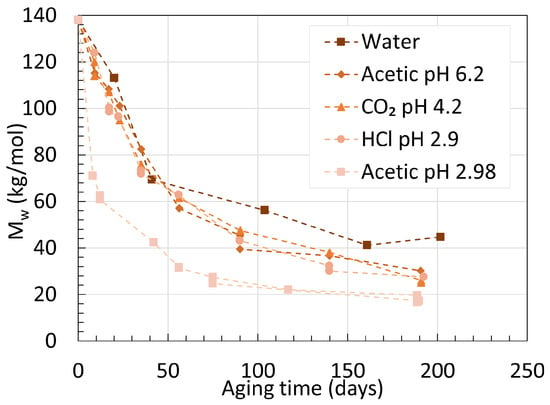
Figure 11. Variations of Mw for stabilized PA11 in different acidic conditions at 100 ∘C. Data from the second work of Hocker et al. [35].
The Mw of PA11 dropped faster and reached lower values when aged in acetic acid than hydrochloric acid, despite their similar pH. Plasticized PA11 resists well against the effect of degradation due to BBSA disrupting the hydrogen bonding matrix in PA11.
References
- Romão, W.; Castro, E.R.; Filho, E.A.S.; Guimarães, R.; Silva, A.L.N.; Teixeira, S.; Paoli, M.; Sena, G.L. Ageing of polyamide 11 used in the manufacture of flexible piping. J. Appl. Polym. Sci. 2009, 114, 1777–1783.
- El-Mazry, C.; Correc, O.; Colin, X. A new kinetic model for predicting polyamide 6-6 hydrolysis and its mechanical embrittlement. Polym. Degrad. Stab. 2012, 97, 1049–1059.
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A material introduction and overview for biomedical applications. Polym. Adv. Technol. 2021, 32, 3368–3383.
- Zhang, Y.; Wang, C.; Yi, Y.; Wang, W.; Yang, J. Synthesis and Properties of Polyamide 6 Random Copolymers Containing an Aromatic Imide Structure. Polymers 2023, 15, 2812.
- Shamey, R.; Sinha, K. A review of degradation of nylon 6. 6 as a result of exposure to environmental conditions. Color. Technol. 2003, 33, 93–107.
- Varghese, M.; Grinstaff, M.W. Beyond nylon 6: Polyamides via ring opening polymerization of designer lactam monomers for biomedical applications. Chem. Soc. Rev. 2022, 51, 8258–8275.
- Grigg, M.N. Thermo-Oxidative Degradation of Polyamide 6. Ph.D. Thesis, School of Physical and Chemical Sciences Queensland University of Technology, Brisbane, Australia, 2006.
- Gonçalves, E.S.; Poulsen, L.; Ogilby, P.R. Mechanism of the temperature-dependent degradation of polyamide 66 films exposed to water. Polym. Degrad. Stab. 2007, 92, 1977–1985.
- Holland, B.J.; Hay, J.N. Thermal degradation of nylon polymers. Polym. Int. 2000, 49, 943–948.
- Ullah, I.; Gul, T.; Ali, M.; Khan, I.; Khan, W.; Asghar, H.; Saeed, K. Preparation, Analysis and UV-Accelerated Photocatalytic Degradation of Pesticide Over Mg Doped ZnO/Nylon 6,6/PMMA Ternary Blend. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3441–3453.
- Gröning, M.; Hakkarainen, M. Headspace solid-phase microextraction–a tool for new insights into the long-term thermo-oxidation mechanism of polyamide 6.6. J. Chromatogr. A 2001, 932, 1–11.
- Vasanthan, N.; Murthy, N.; Bray, R. Investigation of Brill transition in nylon 6 and nylon 6,6 by infrared spectroscopy. Macromolecules 1998, 31, 8433–8435.
- Okamba-Diogo, O.; Richaud, E.; Verdu, J.; Fernagut, F.; Guilment, J.; Fayolle, B. Molecular and macromolecular structure changes in polyamide 11 during thermal oxidation-Kinetic modeling. Polym. Degrad. Stab. 2014, 108, 123–132.
- Krejsa, M.; Udipi, K.; Middleton, J. NMR Analysis of UV- and Heat-Aged Nylon-6,6. Macromolecules 1997, 30, 4695–4703.
- Sanders, B.; Cant, E.; Amel, H.; Jenkins, M. The Effect of Physical Aging and Degradation on the Re-Use of Polyamide 12 in Powder Bed Fusion. Polymers 2022, 14, 2682.
- Moore, R. The photochemical degradation of polyamides and related model N-alkylamides. Polymer 1963, 4, 493–513.
- Levchik, S.; Weil, E.; Lewin, M. Thermal decomposition of aliphatic nylons. Polym. Int. 1999, 48, 532–557.
- Mazry, C.E.; Hassine, M.B.; Correc, O.; Colin, X. Thermal oxidation kinetics of additive free polyamide 6-6. Polym. Degrad. Stab. 2013, 98, 22–36.
- Forsström, D.; Terselius, B. Thermo oxidative stability of polyamide 6 films. I. Mechanical and chemical characterisation. Polym. Degrad. Stab. 2000, 67, 69–78.
- Esmizadeh, E.; Vahidifar, A.; Shojaie, S.; Naderi, G.; Kalaei, M.R.; Mekonnen, T.H. Tailoring the properties of PA6 into high-performance thermoplastic elastomer: Simultaneous reinforcement and impact property modification. Mater. Today Commun. 2021, 26, 102027.
- Arhant, M.; Lolive, E.; Bonnemains, T.; Davies, P. A study of pure hydrolysis of carbon fibre reinforced polyamide 6 composites tested under mode I loading. Compos. Part A Appl. Sci. Manuf. 2021, 152, 106719.
- Deshoulles, Q.; Le Gall, M.; Dreanno, C.; Arhant, M.; Priour, D.; Le Gac, P. Chemical coupling between oxidation and hydrolysis in polyamide 6-A key aspect in the understanding of microplastic formation. Polym. Degrad. Stab. 2022, 197, 109851.
- Deshoulles, Q.; Gall, M.M.L.; Dreanno, C.; Arhant, M.; Priour, D.; Gac, P.Y.L. Modelling pure polyamide 6 hydrolysis: Influence of water content in the amorphous phase. Polym. Degrad. Stab. 2021, 183, 109435.
- Zhang, C.; Liu, C.; Zhao, H.; Hu, W.; Liu, G.; Zhao, Y.; Dong, X.; Wang, K.; Zhang, J.; Li, X.; et al. Effect of nanoparticle and glass fiber on the hydrothermal aging of polyamide 6. J. Appl. Polym. Sci. 2020, 137, 49585.
- Deshoulles, Q.; Gall, M.M.L.; Dreanno, C.; Arhant, M.; Stoclet, G.; Priour, D.; Gac, P.L. Origin of embrittlement in Polyamide 6 induced by chemical degradations: Mechanisms and governing factors. Polym. Degrad. Stab. 2021, 191, 109657.
- Costa, D.R.; Costa, M.F.; Grytten, F. Morphological changes of polyamide 11 through the corrected inherent viscosity plateau. J. Appl. Polym. Sci. 2022, 139, 52223.
- Latko-Durałek, P.; Kolbuk, D.; Kozera, R.; Boczkowska, A. Microstructural Characterization and Mechanical Properties of PA11 Nanocomposite Fibers. J. Mater. Eng. Perform. 2015, 25, 68–75.
- Meyer, A.; Jones, N.; Lin, Y.; Kranbuehl, D.E. Characterizing and Modeling the Hydrolysis of Polyamide-11 in a pH 7 Water Environment. Macromolecules 2002, 44, 6531–6536.
- Hocker, S.J.; Hudson-Smith, N.V.; Smith, P.T.; Komatsu, C.H.; Dickinson, L.R.; Schniepp, H.C.; Kranbuehl, D.E. Graphene oxide reduces the hydrolytic degradation in polyamide-11. Polymer 2017, 126, 248–258.
- Jacques, B.; Werth, M.; Merdas, I.; Thominette, F.; Verdu, J. Hydrolytic ageing of polyamide 11. 1. Hydrolysis kinetics in water. Polymer 2002, 43, 6439–6447.
- Maïza, S.; Lefebvre, X.; Brusselle-Dupend, N.; Klopffer, M.H.; Cangémi, L.; Castagnet, S.; Grandidier, J.C. Physicochemical and mechanical degradation of polyamide 11 induced by hydrolysis and thermal aging. J. Appl. Polym. Sci. 2019, 136, 47628.
- Chaupart, N.; Serpe, G.; Verdu, J. Molecular weight distribution and mass changes during polyamide hydrolysis. Polymer 1998, 39, 1375–1380.
- Abastari; Sakai, T.; Sembokuya, H.; Kubouchi, M.; Tsuda, K. Study on permeation behavior and chemical degradation of PA66 in acid solution. Polym. Degrad. Stab. 2007, 39, 1375–1380.
- Hocker, S.; Rhudy, A.K.; Ginsburg, G.; Kranbuehl, D.E. Polyamide hydrolysis accelerated by small weak organic acids. Polymer 2014, 55, 5057–5064.
- Hocker, S.J.; Kim, W.T.; Schniepp, H.C.; Kranbuehl, D.E. Polymer crystallinity and the ductile to brittle transition. Polymer 2018, 158, 72–76.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
4 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





