
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | WAEL JALLOUL | -- | 2551 | 2023-12-05 06:19:44 | | | |
| 2 | Peter Tang | Meta information modification | 2551 | 2023-12-05 07:07:01 | | |
Video Upload Options
α radioisotopes can offer a treatment choice to individuals who are not responding to β− or gamma-radiation therapy or chemotherapy drugs. Only a few α-particle emitters are suitable for targeted alpha therapy (TAT) and clinical applications. The majority of available clinical research involves 225Ac and its daughter nuclide 213Bi. Additionally, the 225Ac disintegration cascade generates γ decays that can be used in single-photon emission computed tomography (SPECT) imaging, expanding the potential theranostic applications in nuclear medicine. Despite the growing interest in applying 225Ac, the restricted global accessibility of this radioisotope makes it difficult to conduct extensive clinical trials for many radiopharmaceutical candidates.
1. Introduction
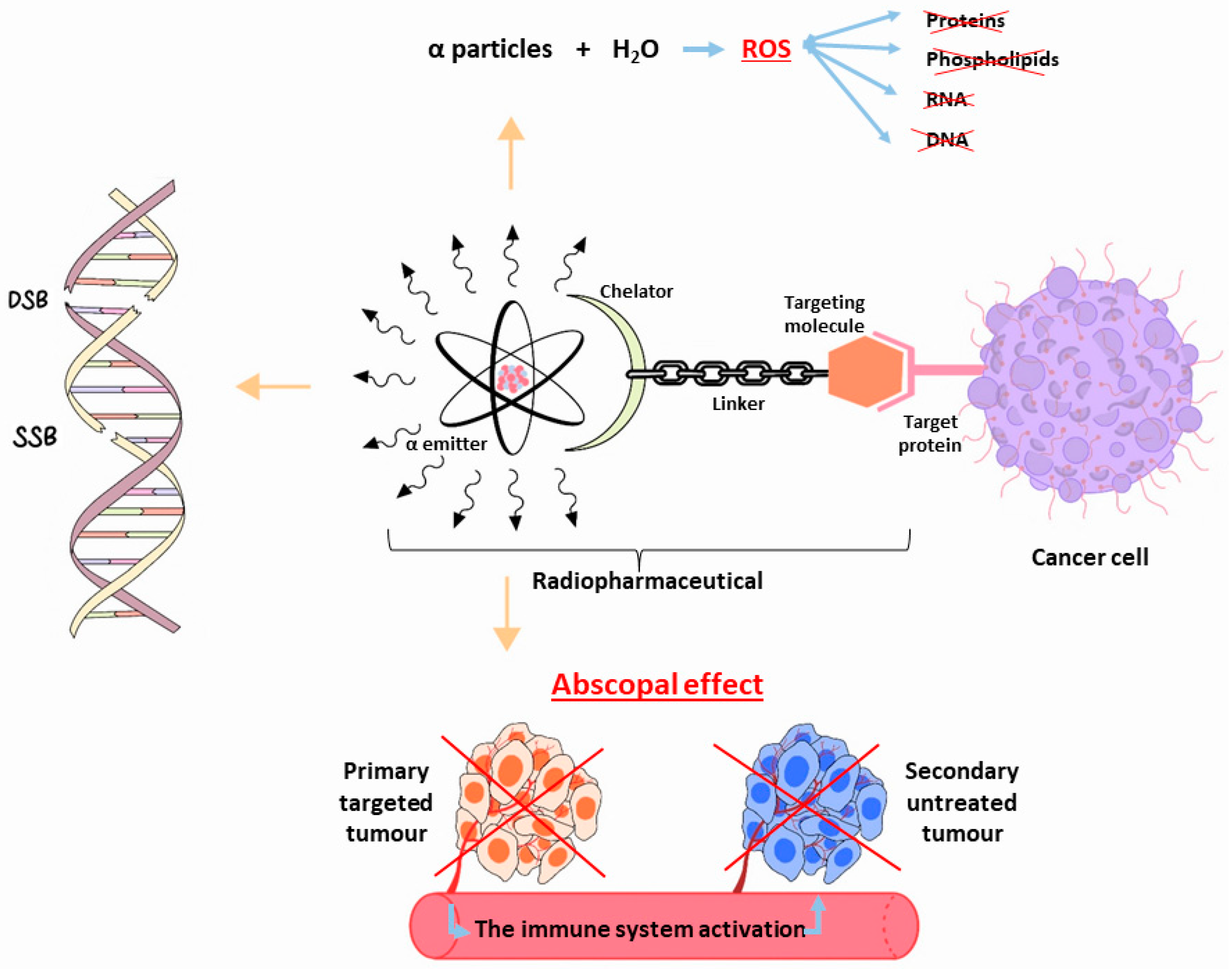
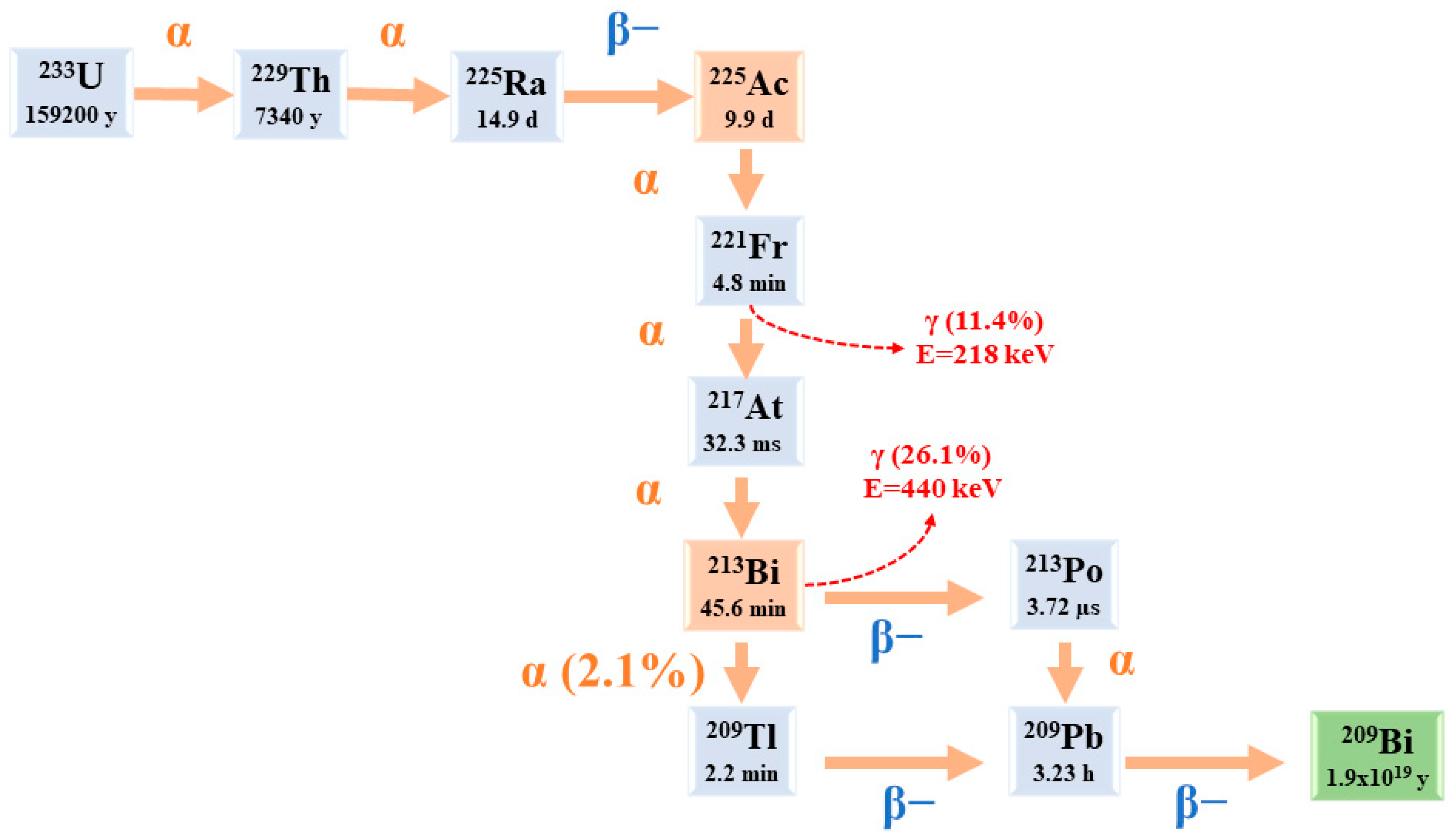
2. 225Ac: Physical Characteristics
3. 225Ac and Its Potential Theranostic Use
4. Radiochemistry
|
Study |
Preparation Method |
Radiopharmaceutical |
RCY/RCP |
|---|---|---|---|
|
Abou. et al., 2022 [41] |
|
225Ac-DOTA-conjugated peptide |
>99%/>95% |
|
Dumond. et al., 2022 [42] |
|
225Ac-PSMA-617 |
>99%/98 ± 1% |
|
Thakral. et al., 2021 [43] |
|
225Ac-PSMA-617 |
85–87%/97–99% |
|
Kelly. et al., 2021 [44] |
|
225Ac-PSMA conjugated peptide/ 225Ac-DOTA conjugated peptide/ 225Ac-macropa conjugated peptide |
2.7 ± 0.55%–98.8 ± 0.09%/1.8–99.5% |
|
Hooijman. et al., 2021 [45] |
|
225Ac-PSMA-I&T |
>95%/>90% |
5. 225Ac Radiopharmaceuticals and Clinical Applications
|
Disease |
Study |
Radiopharmaceutical |
|---|---|---|
|
Prostate cancer |
Parida et al., 2023 [53] |
225Ac-PSMA RLT |
|
Ma et al., 2022 [54] |
225Ac-PSMA-617 |
|
|
Sanli et al., 2021 [55] |
225Ac-PSMA-617 |
|
|
Sen et al., 2021 [56] |
225Ac-PSMA-617 |
|
|
Zacherl et al., 2021 [50] |
225Ac-PSMA-I&T |
|
|
Feuerecker et al., 2021 [57] |
225Ac-PSMA-617 |
|
|
Van Der Doelen et al., 2021 [58] |
225Ac-PSMA-617 |
|
|
Sathekge et al., 2020 [51] |
225Ac-PSMA-617 |
|
|
Yadav et al., 2020 [59] |
225Ac-PSMA-617 |
|
|
Satapathy et al., 2020 [60] |
225Ac-PSMA-617 |
|
|
Sathekge et al., 2019 [61] |
225Ac-PSMA-617 |
|
|
Kratochwil et al., 2018 [62] |
225Ac-PSMA-617 |
|
|
Neuroendocrine tumours |
Ballal et al., 2022 [63] |
225Ac-DOTATATE |
|
Yadav et al., 2022 [48] |
225Ac-DOTATATE |
|
|
Kratochwil et al., 2021 [64] |
225Ac-DOTATATE |
|
|
Ballal et al., 2020 [65] |
225Ac-DOTATATE |
|
|
Kratochwil et al., 2015 [66] |
225Ac-DOTATOC |
|
|
Acute myeloid leukaemia |
Rosenblat et al., 2022 [67] |
225Ac-lintuzumab |
|
Jurcic, 2018 [68] |
225Ac-lintuzumab |
|
|
Jurcic et al., 2016 [69] |
225Ac-lintuzumab |
|
|
Jurcic et al., 2011 [70] |
225Ac-lintuzumab |
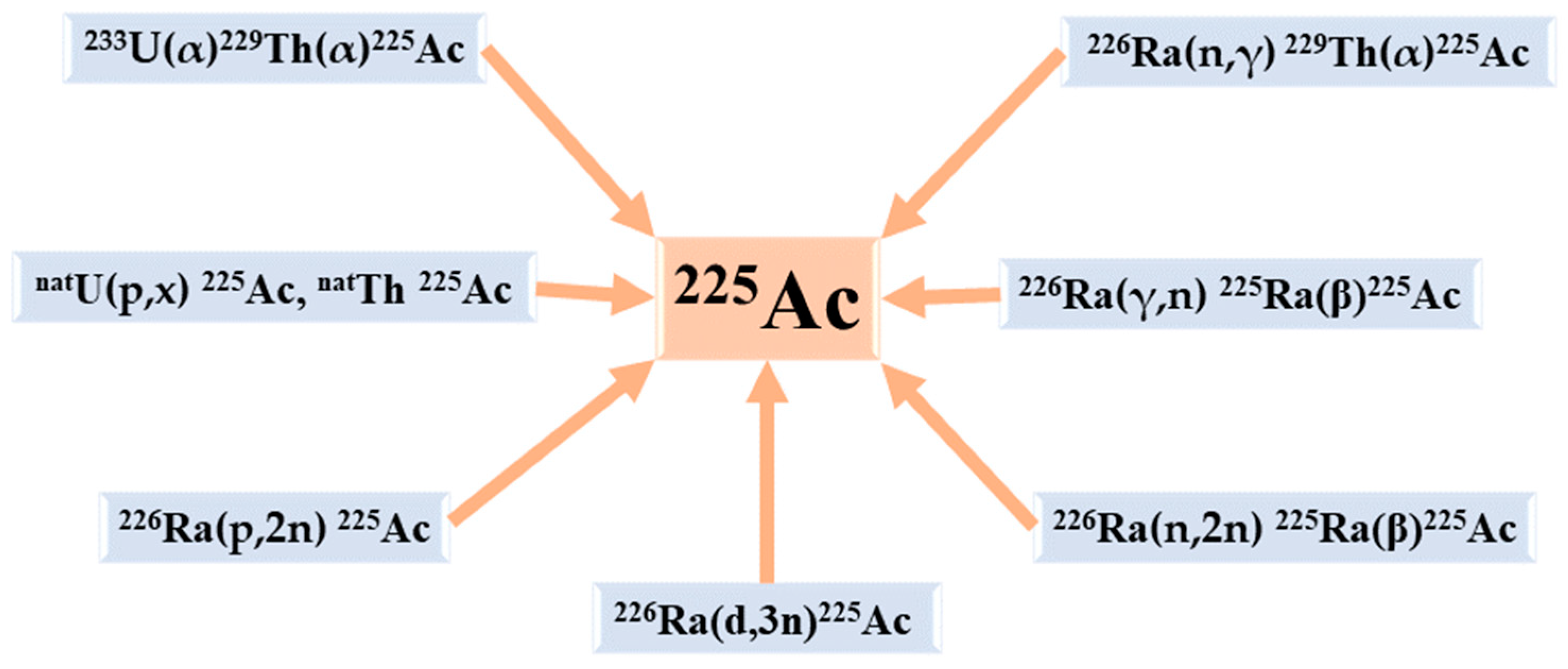
6. The Production Routes of 225Ac
References
- McDevitt, M.R.; Sgouros, G.; Sofou, S. Targeted and Nontargeted α-Particle Therapies. Annu. Rev. Biomed. Eng. 2018, 20, 73–93.
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223.
- Jurcic, J.G.; Ravandi, F.; Pagel, J.M.; Park, J.H.; Smith, B.D.; Douer, D.; Estey, E.H.; Kantarjian, H.M.; Wahl, R.L.; Earle, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy Using Actinium-225 (225Ac)-Lintuzumab (Anti-CD33) in Combination with Low-Dose Cytarabine (LDAC) for Older Patients with Untreated Acute Myeloid Leukemia (AML). Blood 2014, 124, 5293.
- Johnson, J.D.; Heines, M.; Bruchertseifer, F.; Chevallay, E.; Cocolios, T.E.; Dockx, K.; Duchemin, C.; Heinitz, S.; Heinke, R.; Hurier, S.; et al. Resonant Laser Ionization and Mass Separation of 225Ac. Sci. Rep. 2023, 13, 1347.
- Morgenstern, A.; Apostolidis, C.; Kratochwil, C.; Sathekge, M.; Krolicki, L.; Bruchertseifer, F. An Overview of Targeted Alpha Therapy with 225Actinium and 213Bismuth. Curr. Radiopharm. 2018, 11, 200–208.
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (Abridged): Radiobiology and Dosimetry of Alpha-Particle Emitters for Targeted Radionuclide Therapy. J. Nucl. Med. 2010, 51, 311–328.
- Wulbrand, C.; Seidl, C.; Gaertner, F.C.; Bruchertseifer, F.; Morgenstern, A.; Essler, M.; Senekowitsch-Schmidtke, R. Alpha-Particle Emitting 213Bi-Anti-EGFR Immunoconjugates Eradicate Tumor Cells Independent of Oxygenation. PLoS ONE 2013, 8, e64730.
- Elgqvist, J.; Frost, S.; Pouget, J.-P.; Albertsson, P. The Potential and Hurdles of Targeted Alpha Therapy—Clinical Trials and Beyond. Front. Oncol. 2014, 3, 324.
- Friesen, C.; Glatting, G.; Koop, B.; Schwarz, K.; Morgenstern, A.; Apostolidis, C.; Debatin, K.-M.; Reske, S.N. Breaking Chemoresistance and Radioresistance with Anti-CD45 Antibodies in Leukemia Cells. Cancer Res. 2007, 67, 1950–1958.
- Kratochwil, C.; Giesel, F.L.; Bruchertseifer, F.; Mier, W.; Apostolidis, C.; Boll, R.; Murphy, K.; Haberkorn, U.; Morgenstern, A. 213Bi-DOTATOC Receptor-Targeted Alpha-Radionuclide Therapy Induces Remission in Neuroendocrine Tumours Refractory to Beta Radiation: A First-in-Human Experience. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 2106–2119.
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944.
- Humm, J.L.; Cobb, L.M. Nonuniformity of Tumor Dose in Radioimmunotherapy. J. Nucl. Med. 1990, 31, 75–83.
- Guerra Liberal, F.D.C.; O’Sullivan, J.M.; McMahon, S.J.; Prise, K.M. Targeted Alpha Therapy: Current Clinical Applications. Cancer Biother. Radiopharm. 2020, 35, 404–417.
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for Targeted Radionuclide Therapy: From Atom to Bedside. Pharmaceutics 2021, 13, 599.
- Vermeulen, K.; Vandamme, M.; Bormans, G.; Cleeren, F. Design and Challenges of Radiopharmaceuticals. Semin. Nucl. Med. 2019, 49, 339–356.
- Beyls, C.; Haustermans, K.; Deroose, C.M.; Pans, S.; Vanbeckevoort, D.; Verslype, C.; Dekervel, J. Could Autoimmune Disease Contribute to the Abscopal Effect in Metastatic Hepatocellular Carcinoma? Hepatology 2020, 72, 1152–1154.
- Seidl, C. Radioimmunotherapy with α-Particle-Emitting Radionuclides. Immunotherapy 2014, 6, 431–458.
- Zimmermann, R. Is Actinium Really Happening? J. Nucl. Med. 2023, 64, 1516–1518.
- Engle, J.W. The Production of Ac-225. Curr. Radiopharm. 2018, 11, 173–179.
- Hatcher-Lamarre, J.L.; Sanders, V.A.; Rahman, M.; Cutler, C.S.; Francesconi, L.C. Alpha Emitting Nuclides for Targeted Therapy. Nucl. Med. Biol. 2021, 92, 228–240.
- Eychenne, R.; Chérel, M.; Haddad, F.; Guérard, F.; Gestin, J.-F. Overview of the Most Promising Radionuclides for Targeted Alpha Therapy: The “Hopeful Eight”. Pharmaceutics 2021, 13, 906.
- Pommé, S.; Marouli, M.; Suliman, G.; Dikmen, H.; Van Ammel, R.; Jobbágy, V.; Dirican, A.; Stroh, H.; Paepen, J.; Bruchertseifer, F.; et al. Measurement of the 225Ac Half-Life. Appl. Radiat. Isot. 2012, 70, 2608–2614.
- Suliman, G.; Pommé, S.; Marouli, M.; Van Ammel, R.; Stroh, H.; Jobbágy, V.; Paepen, J.; Dirican, A.; Bruchertseifer, F.; Apostolidis, C.; et al. Half-Lives of 221Fr, 217At, 213Bi, 213Po and 209Pb from the 225Ac Decay Series. Appl. Radiat. Isot. 2013, 77, 32–37.
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49.
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in Targeted Alpha-Particle Therapeutic Applications. Curr. Radiopharm. 2011, 4, 306–320.
- Muslimov, A.R.; Antuganov, D.; Tarakanchikova, Y.V.; Karpov, T.E.; Zhukov, M.V.; Zyuzin, M.V.; Timin, A.S. An Investigation of Calcium Carbonate Core-Shell Particles for Incorporation of 225Ac and Sequester of Daughter Radionuclides: In Vitro and in Vivo Studies. J. Control Release 2021, 330, 726–737.
- Nelson, B.J.B.; Wilson, J.; Andersson, J.D.; Wuest, F. Theranostic Imaging Surrogates for Targeted Alpha Therapy: Progress in Production, Purification, and Applications. Pharmaceuticals 2023, 16, 1622.
- Saini, S.; Bartels, J.L.; Appiah, J.-P.K.; Rider, J.H.; Baumhover, N.; Schultz, M.K.; Lapi, S.E. Optimized Methods for the Production of High-Purity 203Pb Using Electroplated Thallium Targets. J. Nucl. Med. 2023, 64, 1791–1797.
- Bobba, K.N.; Bidkar, A.P.; Meher, N.; Fong, C.; Wadhwa, A.; Dhrona, S.; Sorlin, A.; Bidlingmaier, S.; Shuere, B.; He, J.; et al. Evaluation of 134Ce/134La as a PET Imaging Theranostic Pair for 225Ac α-Radiotherapeutics. J. Nucl. Med. 2023, 64, 1076–1082.
- Aluicio-Sarduy, E.; Barnhart, T.E.; Weichert, J.; Hernandez, R.; Engle, J.W. Cyclotron-Produced 132La as a PET Imaging Surrogate for Therapeutic 225Ac. J. Nucl. Med. 2021, 62, 1012–1015.
- Nelson, B.J.B.; Ferguson, S.; Wuest, M.; Wilson, J.; Duke, M.J.M.; Richter, S.; Soenke-Jans, H.; Andersson, J.D.; Juengling, F.; Wuest, F. First In Vivo and Phantom Imaging of Cyclotron-Produced 133La as a Theranostic Radionuclide for 225Ac and 135La. J. Nucl. Med. 2022, 63, 584–590.
- Bailey, T.A.; Mocko, V.; Shield, K.M.; An, D.D.; Akin, A.C.; Birnbaum, E.R.; Brugh, M.; Cooley, J.C.; Engle, J.W.; Fassbender, M.E.; et al. Developing the 134Ce and 134La Pair as Companion Positron Emission Tomography Diagnostic Isotopes for 225Ac and 227Th Radiotherapeutics. Nat. Chem. 2021, 13, 284–289.
- Bailey, T.A.; Wacker, J.N.; An, D.D.; Carter, K.P.; Davis, R.C.; Mocko, V.; Larrabee, J.; Shield, K.M.; Lam, M.N.; Booth, C.H.; et al. Evaluation of 134Ce as a PET Imaging Surrogate for Antibody Drug Conjugates Incorporating 225Ac. Nucl. Med. Biol. 2022, 110–111, 28–36.
- Hu, A.; Aluicio-Sarduy, E.; Brown, V.; MacMillan, S.N.; Becker, K.V.; Barnhart, T.E.; Radchenko, V.; Ramogida, C.F.; Engle, J.W.; Wilson, J.J. Py-Macrodipa: A Janus Chelator Capable of Binding Medicinally Relevant Rare-Earth Radiometals of Disparate Sizes. J. Am. Chem. Soc. 2021, 143, 10429–10440.
- Thiele, N.A.; Brown, V.; Kelly, J.M.; Amor-Coarasa, A.; Jermilova, U.; MacMillan, S.N.; Nikolopoulou, A.; Ponnala, S.; Ramogida, C.F.; Robertson, A.K.H.; et al. An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 14712–14717.
- Rizk, H.E.; Breky, M.M.E.; Attallah, M.F. Development of Purification of No-Carrier-Added 47Sc of Theranostic Interest: Selective Separation Study from the natTi(n,p) Process. Radiochim. Acta 2023, 111, 273–282.
- Mousa, A.M.; Abdel Aziz, O.A.; Al-Hagar, O.E.A.; Gizawy, M.A.; Allan, K.F.; Attallah, M.F. Biosynthetic New Composite Material Containing CuO Nanoparticles Produced by Aspergillus Terreus for 47Sc Separation of Cancer Theranostics Application from Irradiated Ca Target. Appl. Radiat. Isot. 2020, 166, 109389.
- Attallah, M.F.; Rizk, S.E.; Shady, S.A. Separation of 152+154Eu, 90Sr from Radioactive Waste Effluent Using Liquid–Liquid Extraction by Polyglycerol Phthalate. Nucl. Sci. Tech. 2018, 29, 84.
- Hooijman, E.L.; Ntihabose, C.M.; Reuvers, T.G.A.; Nonnekens, J.; Aalbersberg, E.A.; van de Merbel, J.R.J.P.; Huijmans, J.E.; Koolen, S.L.W.; Hendrikx, J.J.M.A.; de Blois, E. Radiolabeling and Quality Control of Therapeutic Radiopharmaceuticals: Optimization, Clinical Implementation and Comparison of Radio-TLC/HPLC Analysis, Demonstrated by Lu-PSMA. EJNMMI Radiopharm. Chem. 2022, 7, 29.
- Mdanda, S.; Ngema, L.M.; Mdlophane, A.; Sathekge, M.M.; Zeevaart, J.R. Recent Innovations and Nano-Delivery of Actinium-225: A Narrative Review. Pharmaceutics 2023, 15, 1719.
- Abou, D.S.; Zerkel, P.; Robben, J.; McLaughlin, M.; Hazlehurst, T.; Morse, D.; Wadas, T.J.; Pandya, D.N.; Oyama, R.; Gaehle, G.; et al. Radiopharmaceutical Quality Control Considerations for Accelerator-Produced Actinium Therapies. Cancer Biother. Radiopharm. 2022, 37, 355–363.
- Dumond, A.R.S.; Rodnick, M.E.; Piert, M.R.; Scott, P.J.H. Synthesis of 225Ac-PSMA-617 for Preclinical Use. Curr. Radiopharm. 2022, 15, 96–103.
- Thakral, P.; Simecek, J.; Marx, S.; Kumari, J.; Pant, V.; Sen, I.B. In-House Preparation and Quality Control of Ac-225 Prostate-Specific Membrane Antigen-617 for the Targeted Alpha Therapy of Castration-Resistant Prostate Carcinoma. Indian. J. Nucl. Med. 2021, 36, 114–119.
- Kelly, J.M.; Amor-Coarasa, A.; Sweeney, E.; Wilson, J.J.; Causey, P.W.; Babich, J.W. A Suitable Time Point for Quantifying the Radiochemical Purity of 225Ac-Labeled Radiopharmaceuticals. EJNMMI Radiopharm. Chem. 2021, 6, 38.
- Hooijman, E.L.; Chalashkan, Y.; Ling, S.W.; Kahyargil, F.F.; Segbers, M.; Bruchertseifer, F.; Morgenstern, A.; Seimbille, Y.; Koolen, S.L.W.; Brabander, T.; et al. Development of Ac-PSMA-I&T for Targeted Alpha Therapy According to GMP Guidelines for Treatment of mCRPC. Pharmaceutics 2021, 13, 715.
- Busslinger, S.D.; Tschan, V.J.; Richard, O.K.; Talip, Z.; Schibli, R.; Müller, C. Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with Ac-PSMA-617. Cancers 2022, 14, 5651.
- King, A.P.; Gutsche, N.T.; Raju, N.; Fayn, S.; Baidoo, K.E.; Bell, M.M.; Olkowski, C.S.; Swenson, R.E.; Lin, F.I.; Sadowski, S.M.; et al. 225Ac-MACROPATATE: A Novel α-Particle Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. J. Nucl. Med. 2023, 64, 549–554.
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Bal, C. Efficacy and Safety of 225Ac-DOTATATE Targeted Alpha Therapy in Metastatic Paragangliomas: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1595–1606.
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First Patient Exceeding 5-Year Complete Remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312.
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674.
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.O.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2020, 61, 62–69.
- Camacaro, J.F.; Dunckley, C.P.; Harman, S.E.; Fitzgerald, H.A.; Lakes, A.L.; Liao, Z.; Ludwig, R.C.; McBride, K.M.; Yalcintas Bethune, E.; Younes, A.; et al. Development of 225Ac Production from Low Isotopic Dilution 229Th. ACS Omega 2023, 8, 38822–38827.
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191.
- Ma, J.; Li, L.; Liao, T.; Gong, W.; Zhang, C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 796657.
- Sanli, Y.; Kuyumcu, S.; Simsek, D.H.; Büyükkaya, F.; Civan, C.; Isik, E.G.; Ozkan, Z.G.; Basaran, M.; Sanli, O. 225Ac-Prostate-Specific Membrane Antigen Therapy for Castration-Resistant Prostate Cancer: A Single-Center Experience. Clin. Nucl. Med. 2021, 46, 943–951.
- Sen, I.; Thakral, P.; Tiwari, P.; Pant, V.; Das, S.S.; Manda, D.; Raina, V. Therapeutic Efficacy of 225Ac-PSMA-617 Targeted Alpha Therapy in Patients of Metastatic Castrate Resistant Prostate Cancer after Taxane-Based Chemotherapy. Ann. Nucl. Med. 2021, 35, 794–810.
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-Resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350.
- Van der Doelen, M.J.; Mehra, N.; van Oort, I.M.; Looijen-Salamon, M.G.; Janssen, M.J.R.; Custers, J.A.E.; Slootbeek, P.H.J.; Kroeze, L.I.; Bruchertseifer, F.; Morgenstern, A.; et al. Clinical Outcomes and Molecular Profiling of Advanced Metastatic Castration-Resistant Prostate Cancer Patients Treated with 225Ac-PSMA-617 Targeted Alpha-Radiation Therapy. Urol. Oncol. 2021, 39, 729.e7–729.e16.
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and Safety of 225Ac-PSMA-617 Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer Patients. Theranostics 2020, 10, 9364–9377.
- Satapathy, S.; Mittal, B.R.; Sood, A.; Das, C.K.; Singh, S.K.; Mavuduru, R.S.; Bora, G.S. Health-Related Quality-of-Life Outcomes with Actinium-225-Prostate-Specific Membrane Antigen-617 Therapy in Patients with Heavily Pretreated Metastatic Castration-Resistant Prostate Cancer. Indian. J. Nucl. Med. 2020, 35, 299–304.
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in Chemotherapy-Naive Patients with Advanced Prostate Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138.
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802.
- Ballal, S.; Yadav, M.P.; Tripathi, M.; Sahoo, R.K.; Bal, C. Survival Outcomes in Metastatic Gastroenteropancreatic Neuroendocrine Tumor Patients Receiving Concomitant 225Ac-DOTATATE Targeted Alpha Therapy and Capecitabine: A Real-World Scenario Management Based Long-Term Outcome Study. J. Nucl. Med. 2022, 64, 211–218.
- Kratochwil, C.; Apostolidis, L.; Rathke, H.; Apostolidis, C.; Bicu, F.; Bruchertseifer, F.; Choyke, P.L.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Dosing 225Ac-DOTATOC in Patients with Somatostatin-Receptor-Positive Solid Tumors: 5-Year Follow-up of Hematological and Renal Toxicity. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 54–63.
- Ballal, S.; Yadav, M.P.; Bal, C.; Sahoo, R.K.; Tripathi, M. Broadening Horizons with 225Ac-DOTATATE Targeted Alpha Therapy for Gastroenteropancreatic Neuroendocrine Tumour Patients Stable or Refractory to 177Lu-DOTATATE PRRT: First Clinical Experience on the Efficacy and Safety. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 934–946.
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. Ac-225-DOTATOC—An Empiric Dose Finding for Alpha Particle Emitter Based Radionuclide Therapy of Neuroendocrine Tumors. J. Nucl. Med. 2015, 56, 1232.
- Rosenblat, T.L.; McDevitt, M.R.; Carrasquillo, J.A.; Pandit-Taskar, N.; Frattini, M.G.; Maslak, P.G.; Park, J.H.; Douer, D.; Cicic, D.; Larson, S.M.; et al. Treatment of Patients with Acute Myeloid Leukemia with the Targeted Alpha-Particle Nanogenerator Actinium-225-Lintuzumab. Clin. Cancer Res. 2022, 28, 2030–2037.
- Jurcic, J.G. Clinical Studies with Bismuth-213 and Actinium-225 for Hematologic Malignancies. Curr. Radiopharm. 2018, 11, 192–199.
- Jurcic, J.G.; Levy, M.Y.; Park, J.H.; Ravandi, F.; Perl, A.E.; Pagel, J.M.; Smith, B.D.; Estey, E.H.; Kantarjian, H.; Cicic, D.; et al. Phase I Trial of Targeted Alpha-Particle Therapy with Actinium-225 (225Ac)-Lintuzumab and Low-Dose Cytarabine (LDAC) in Patients Age 60 or Older with Untreated Acute Myeloid Leukemia (AML). Blood 2016, 128, 4050.
- Jurcic, J.G.; Rosenblat, T.L.; McDevitt, M.R.; Pandit-Taskar, N.; Carrasquillo, J.A.; Chanel, S.M.; Zikaras, K.; Frattini, M.G.; Maslak, P.G.; Cicic, D.; et al. Phase I Trial of the Targeted Alpha-Particle Nano-Generator Actinium-225 (225Ac)-Lintuzumab (Anti-CD33; HuM195) in Acute Myeloid Leukemia (AML). Blood 2011, 118, 768.
- Pretze, M.; Kunkel, F.; Runge, R.; Freudenberg, R.; Braune, A.; Hartmann, H.; Schwarz, U.; Brogsitter, C.; Kotzerke, J. Ac-EAZY! Towards GMP-Compliant Module Syntheses of 225Ac-Labeled Peptides for Clinical Application. Pharmaceuticals 2021, 14, 652.
- Eryilmaz, K.; Kilbas, B. Fully-Automated Synthesis of 177Lu Labelled FAPI Derivatives on the Module Modular Lab-Eazy. EJNMMI Radiopharm. Chem. 2021, 6, 16.





