2. 225Ac: Physical Characteristics
Actinium is a radioactive component with atomic number 89 [
20]. Only two of its 32 isotopes,
228Ac and
227Ac, are naturally produced as a result of the disintegration of
232Th and
235U, respectively [
20,
21]. With its long half-life of 21.7 years and predominant β− emissions decay,
227Ac represents the most common actinium isotope. However,
228Ac, which is also a β− emitter, is highly uncommon [
20,
21].
225Ac is the initial element in the actinide family, and its radioactive parents are parts of the now-extinct “neptunium series” [
19,
21]. This α-emitter isotope has a long half-life of 9.9 days [
5,
22].
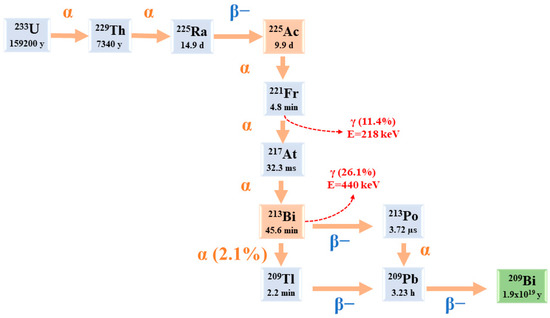
Starting from
225Ac to reach
209Bi (T
1/2 = 1.9 × 10
19 y), the decay series includes six short-lived radionuclide daughters [
5,
23].
This radioactive cascade is represented by
221Fr (T
1/2 = 4.8 min; 6.3 MeV α particle and 218 keV γ emission),
217At (T
1/2 = 32.3 ms; 7.1 MeV α particle),
213Bi (T
1/2 = 45.6 min; 5.9 MeV α particle, 492 keV β− particle and 440 keV γ emission),
213Po (T
1/2 = 3.72 µs; 8.4 MeV α particle),
209Tl (T
1/2 = 2.2 min; 178 keV β− particle),
209Pb (T
1/2 = 3.23 h; 198 keV β− particle) [
24] (
Figure 2) [
14].
Figure 2. The decay chain of 233U to 225Ac and 213Bi.
3. 225Ac and Its Potential Theranostic Use
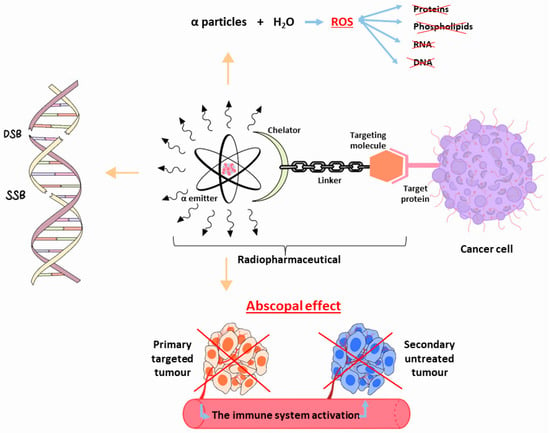
225Ac is considered a “nanogenerator”, since one decay of this element produces a total of four α and three β particles, in addition to two γ emissions [
24]. Taking into account its α particle emissions, along with the fact that the non-tumour binding activity can be eliminated before most of its dose is deposited in organs,
225Ac is considered an appealing choice for TAT [
24,
25]. However, it is important to give attention to the notable
225Ac cytotoxicity, including renal toxicity [
26], due to its extended half-life and the various α particles produced throughout its decay chain [
5].
A theranostic-based approach, characterised by the imaging–therapeutic duality, is the process of obtaining positron emission tomography (PET) and SPECT scans by exchanging the therapeutic α-emitting radionuclide with a positron or gamma diagnostic imaging radionuclide. Significant information on dosimetry and TAT reactions is obtained from these relevant nuclear medicine images.
Chemical characteristics, half-life, radioactive emission type and intensity, related dosimetry, ease and scalability of production, radionuclidic purity, economics, and radionuclide progeny considerations are the factors that determine “the ideal” imaging surrogates for targeted alpha therapy [
27,
28].
Therapeutic use of
225Ac is often paired with imperfect PET imaging surrogates, such as
68Ga,
89Zr, or
111In, despite significant differences in their half-lives or chelation chemistry [
29]. Studies are being conducted to address the limitations of imaging radionuclides by utilising lanthanum (La) as a potential alternative, especially
132La (T
1/2 = 4.8 h, 42% β+) and
133La (T
1/2 = 3.9 h, 7% β+) [
30,
31]. However, the half-lives of these isotopes are much shorter than that of
225Ac, limiting their applicability in PET imaging [
29]. In this regard, the production of
134Ce (T
1/2 = 3.2 d) has recently been started by the U.S. Department of Energy (DOE) Isotope Program [
32]. The long
134Ce T
1/2 and the similar chemical properties of
225Ac and
134Ce were considered potential benefits for monitoring in vivo pharmacokinetics. For PET imaging of the chelate and the antibody trastuzumab,
134Ce has been demonstrated to bind with diethylenetriamine pentaacetate (DTPA) [
32] and dodecane tetraacetic acid (DOTA) [
33]. On the other hand, greater molar ratios and higher temperatures are needed for isotope combinations with DOTA and DTPA [
29]. In contrast, N, N′-bis[(6-carboxy-2-pyridyl)methyl]-4,13-diaza-18-crown-6 (macropa) has shown great stability for nonradioactive cerium and better chelate characteristics for
225Ac [
34], indicating that it might be useful for the theranostic development of
134Ce/
225Ac [
35].
The potential use of γ disintegrations, obtained by the decay of the intermediate
221Fr (218 keV, 11.6% emission probability) and
213Bi (440 keV, 26.1% emission probability) [
5], in SPECT in vivo imaging could lead the
225Ac radioactive cascade to a possible theranostic prospective in nuclear medicine applications. Nonetheless, planar SPECT imaging would be challenging because of the effectiveness of
225Ac, which results in modest administered doses (~50–200 kBq/kg [
5]), along with low γ emissions [
24,
25]. As a possible solution to this limitation, we can notice the suitable use of
213Bi, which can be isolated from the
225Ac decay cascades [
24]. Nevertheless, it is mandatory to consider the short half-life of
213Bi (45.6 min), which poses difficulties for processing, radiolabelling, and radiopharmaceutical delivery [
24]. In addition, it is necessary to point out that these radiations make reaction monitoring complicated. Moreover, the secular equilibrium must be attained (for at least 6 h) before measuring a trustworthy radiochemical yield (RCY) [
21]. Actinium’s chemistry lacks advancement because of its restricted availability; all Ac isotopes need specific management and facilities [
20].
4. Radiochemistry
During the production of radionuclides, it is mandatory to take into consideration a set of important aspects, such as safety, the co-generation of a few long-lived radionuclidic impurities, and adjustability, to enable delivery through clinical sites [
27]. Once the target material has been irradiated, potent chemical purification methods are required to isolate the radioisotope [
27,
36,
37,
38]. Furthermore, the alpha particle may radiolytically damage the radiopharmaceutical itself, reducing in vivo targeting and producing more radioactive deposits in nontarget tissue. [
27].
Since radiopharmaceuticals are considered typical pharmaceuticals, special manuals have been developed in the
European Pharmacopoeia to deal with quality control issues [
39]. Additionally, optimised protocols for preparing
225Ac agents in therapeutic doses have been established [
40] (
Table 1).
Table 1. Research on 225Ac chemistry. RCY = Radiochemical yield, RCP = Radiochemical purity, TLC = Thin-layer chromatography, ITLC = Instant thin-layer chromatography.
5. 225Ac Radiopharmaceuticals and Clinical Applications
The delivery of the radiopharmaceutical via the circulatory system enables the targeting of both the main tumour and its metastases. Whether a radiopharmaceutical is intended for therapeutic or diagnostic purposes depends on the decay properties of the linked radioisotope. For the purpose of curing, controlling, or palliating symptoms, TAT aims to provide an adequate amount of ionising radiation to intended malignities areas [
27]. This means that any TAT agent must have a thorough understanding of its stability, pharmacokinetics, and dosimetry.
Investigations on
225Ac have shown potential in treating neuroendocrine tumours, acute myeloid leukaemia, and metastatic prostate cancer, and more radiopharmaceuticals are being developed for other cancer types [
46,
47,
48,
49,
50,
51,
52] (
Table 2).
Table 2. Clinical research based on 225Ac.
The use of
225Ac in clinical practice is limited by its low availability. Breaking through this barrier would allow
225Ac therapy to spread widely. Automated synthesis and consistent patient doses are essential, regardless of the production route chosen for this α-isotope acquisition.
225Ac can be adapted for the commonly accessible DOTA-conjugated peptides for therapy [
41], which are already capable of labelling
177Lu or
90Y. Marc Pretze et al. [
71] studied the effectiveness and consistency of the radiosynthesis process for creating
225Ac-labelled DOTA-conjugated peptides. Additionally, the research aimed to establish whether this process could be adapted for clinical production purposes through an automated synthesis platform (cassette-based module—Modular-Lab EAZY, Eckert & Ziegler) [
72]. After comparing two purification methods, the researchers obtained
225Ac-labelled peptides in an RCY of 80–90% for tumour therapy in patients [
71]. Thus, the whole process was meticulously validated in accordance with the regulations of the German Pharmaceuticals Act §13.2b, knowing that the estimated costs for the automated synthesis of 1 MBq
225Ac is around EUR 300–390, taking into account that the peptides would cost EUR 600–1000, the cassettes would cost EUR 180–200, and the ML EAZY would cost EUR ~30,000 [
71].
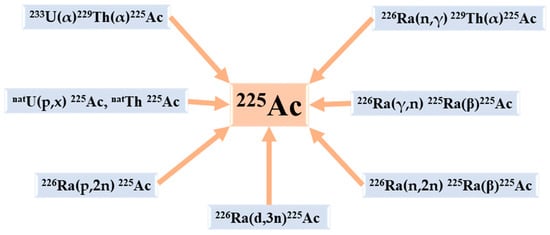
6. The Production Routes of 225Ac
As already mentioned,
225Ac is part of the
237Np disintegration family that has vanished in nature. This radioactive element could be artificially reproduced [
21]. In addition to direct production paths,
225Ac is conveniently reachable at numerous points along the decay chain, in particular via
233U (T
1/2 =159200 y, 100% α),
229Th (T
1/2 = 7340 y, 100% α), and
225Ra (T
1/2 = 14.9 d, 100% β−) [
19].
225Ac possesses many fewer nucleons than other actinide nuclei that are more stable to be employed as production targets, such as
232Th and
226Ra [
19]. Thus, production methods should, with rare exceptions, rely on radioactive decay or greater energy bombardments.
The available production routes of
225Ac and its parents are listed below (
Figure 3) [
14]:
Figure 3. The principal production routes for 225Ac.