
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Thang Phan Nguyen | -- | 3315 | 2023-10-31 11:21:26 | | | |
| 2 | Thang Phan Nguyen | + 6536 word(s) | 9851 | 2023-10-31 11:24:22 | | | | |
| 3 | Lindsay Dong | -6536 word(s) | 3315 | 2023-11-01 02:12:31 | | |
Video Upload Options
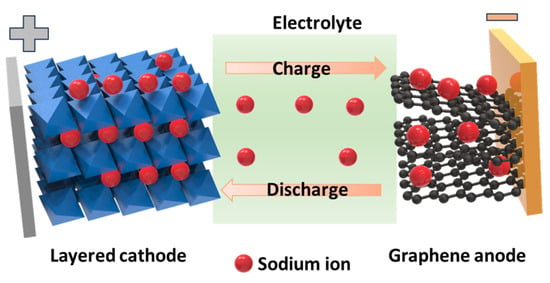
Emerging energy storage systems have received significant attention along with the development of renewable energy, thereby creating a green energy platform for humans. Lithium-ion batteries (LIBs) are commonly used, such as in smartphones, tablets, earphones, and electric vehicles. However, lithium has certain limitations including safety, cost-effectiveness, and environmental issues. Sodium is believed to be an ideal replacement for lithium owing to its infinite abundance, safety, low cost, environmental friendliness, and energy storage behavior similar to that of lithium. Inhered in the achievement in the development of LIBs, sodium-ion batteries (SIBs) have rapidly evolved to be commercialized. Among the cathode, anode, and electrolyte, the cathode remains a significant challenge for achieving a stable, high-rate, and high-capacity device.
1. Introduction
2. SIB Cathode Materials
2.1. Inorganic Compounds
2.1.1. Layered Oxide Materials (NaxMO2)
2.1.2. Tunnel Oxides
2.1.3. Polyanionic Compounds
Phosphate-Based Compound
NASICON
2.1.4. Pyrophosphates
2.1.5. Silicates
2.2. Organic Compounds
2.3. Metal–Organic Compounds: Prussian Blue Analogs
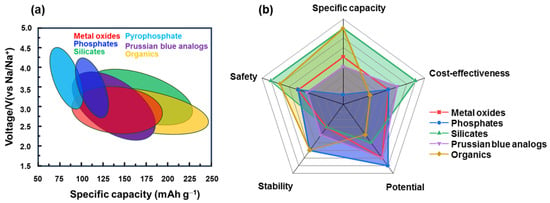
3. Summary
References
- Mahmud, S.; Rahman, M.; Kamruzzaman, M.; Ali, M.O.; Emon, M.S.A.; Khatun, H.; Ali, M.R. Recent advances in lithium-ion battery materials for improved electrochemical performance: A review. Results Eng. 2022, 15, 100472.
- Liu, J.; Bao, Z.N.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.Y.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186.
- Boebinger, M.G.; Yarema, O.; Yarema, M.; Unocic, K.A.; Unocic, R.R.; Wood, V.; McDowell, M.T. Spontaneous and reversible hollowing of alloy anode nanocrystals for stable battery cycling. Nat. Nanotechnol. 2020, 15, 475–481.
- Masias, A.; Marcicki, J.; Paxton, W.A. Opportunities and Challenges of Lithium Ion Batteries in Automotive Applications. ACS Energy Lett. 2021, 6, 621–630.
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683.
- Zhang, W.; Zhang, F.; Ming, F.; Alshareef, H.N. Sodium-ion battery anodes: Status and future trends. EnergyChem 2019, 1, 100012.
- Li, X.F.; Dhanabalan, A.; Gu, L.; Wang, C.L. Three-Dimensional Porous Core-Shell Sn@Carbon Composite Anodes for High-Performance Lithium-Ion Battery Applications. Adv. Energy Mater. 2012, 2, 238–244.
- Hatzell, K.B.; Chen, X.C.; Cobb, C.L.; Dasgupta, N.P.; Dixit, M.B.; Marbella, L.E.; McDowell, M.T.; Mukherjee, P.P.; Verma, A.; Viswanathan, V.; et al. Challenges in Lithium Metal Anodes for Solid-State Batteries. ACS Energy Lett. 2020, 5, 922–934.
- Velumani, D.; Bansal, A. Thermal Behavior of Lithium- and Sodium-Ion Batteries: A Review on Heat Generation, Battery Degradation, Thermal Runway—Perspective and Future Directions. Energy Fuels 2022, 36, 14000–14029.
- Ponnada, S.; Kiai, M.S.; Krishnapriya, R.; Singhal, R.; Sharma, R.K. Lithium-Free Batteries: Needs and Challenges. Energy Fuels 2022, 36, 6013–6026.
- Abraham, K.M. How Comparable Are Sodium-Ion Batteries to Lithium-Ion Counterparts? ACS Energy Lett. 2020, 5, 3544–3547.
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Boorboor Ajdari, F.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505.
- Ellis, B.L.; Nazar, L.F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid. State Mater. Sci. 2012, 16, 168–177.
- Liu, Q.; Zhao, X.; Yang, Q.; Hou, L.; Mu, D.; Tan, G.; Li, L.; Chen, R.; Wu, F. The Progress in the Electrolytes for Solid State Sodium-Ion Battery. Adv. Mater. Technol. 2023, 8, 2200822.
- Åvall, G.; Mindemark, J.; Brandell, D.; Johansson, P. Sodium-Ion Battery Electrolytes: Modeling and Simulations. Adv. Energy Mater. 2018, 8, 1703036.
- Wang, B.; Wang, X.; Liang, C.; Yan, M.; Jiang, Y. An All-Prussian-Blue-Based Aqueous Sodium-Ion Battery. ChemElectroChem 2019, 6, 4848–4853.
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials: A growing research path. Energy Environ. Sci. 2013, 6, 2312–2337.
- Li, Y.; Wu, F.; Li, Y.; Liu, M.; Feng, X.; Bai, Y.; Wu, C. Ether-based electrolytes for sodium ion batteries. Chem. Soc. Rev. 2022, 51, 4484–4536.
- Wang, X.; Roy, S.; Shi, Q.; Li, Y.; Zhao, Y.; Zhang, J. Progress in and application prospects of advanced and cost-effective iron (Fe)-based cathode materials for sodium-ion batteries. J. Mater. Chem. A 2021, 9, 1938–1969.
- Lee, J.M.; Singh, G.; Cha, W.; Kim, S.; Yi, J.; Hwang, S.-J.; Vinu, A. Recent Advances in Developing Hybrid Materials for Sodium-Ion Battery Anodes. ACS Energy Lett. 2020, 5, 1939–1966.
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation “Beyond Li-Ion” Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669.
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614.
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033.
- He, J.; Wei, Y.; Zhai, T.; Li, H. Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries. Mater. Chem. Front. 2018, 2, 437–455.
- Yu, D.Y.W.; Prikhodchenko, P.V.; Mason, C.W.; Batabyal, S.K.; Gun, J.; Sladkevich, S.; Medvedev, A.G.; Lev, O. High-capacity antimony sulphide nanoparticle-decorated graphene composite as anode for sodium-ion batteries. Nat. Commun. 2013, 4, 2922.
- Hwang, J.-Y.; Du, H.-L.; Yun, B.-N.; Jeong, M.-G.; Kim, J.-S.; Kim, H.; Jung, H.-G.; Sun, Y.-K. Carbon-Free TiO2 Microspheres as Anode Materials for Sodium Ion Batteries. ACS Energy Lett. 2019, 4, 494–501.
- Ni, J.; Li, L.; Lu, J. Phosphorus: An Anode of Choice for Sodium-Ion Batteries. ACS Energy Lett. 2018, 3, 1137–1144.
- Durai, L.; Gopalakrishnan, A.; Badhulika, S. Solid-state synthesis of β-NaAlO2 nanoflakes as an anode material for high-performance sodium-ion batteries. Mater. Chem. Front. 2022, 6, 2913–2920.
- Nam, K.-H.; Hwa, Y.; Park, C.-M. Zinc Phosphides as Outstanding Sodium-Ion Battery Anodes. ACS Appl. Mater. Interfaces 2020, 12, 15053–15062.
- Xu, H.; Chen, H.; Gao, C. Advanced Graphene Materials for Sodium/Potassium/Aluminum-Ion Batteries. ACS Mater. Lett. 2021, 3, 1221–1237.
- He, M.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.-P. Assessment of the first commercial Prussian blue based sodium-ion battery. J. Power Sources 2022, 548, 232036.
- Yadav, P.; Shelke, V.; Patrike, A.; Shelke, M. Sodium-based batteries: Development, commercialization journey and new emerging chemistries. Oxf. Open Mater. Sci. 2022, 3, itac019.
- Wang, M.; Wang, Q.; Ding, X.; Wang, Y.; Xin, Y.; Singh, P.; Wu, F.; Gao, H. The prospect and challenges of sodium-ion batteries for low-temperature conditions. Interdiscip. Mater. 2022, 1, 373–395.
- Zhao, L.; Zhang, T.; Li, W.; Li, T.; Zhang, L.; Zhang, X.; Wang, Z. Engineering of Sodium-Ion Batteries: Opportunities and Challenges. Engineering, 2022; in press.
- Xie, J.; Gu, P.; Zhang, Q. Nanostructured Conjugated Polymers: Toward High-Performance Organic Electrodes for Rechargeable Batteries. ACS Energy Lett. 2017, 2, 1985–1996.
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-economics. Acc. Chem. Res. 2023, 56, 284–296.
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364.
- Stansby, J.H.; Sharma, N.; Goonetilleke, D. Probing the charged state of layered positive electrodes in sodium-ion batteries: Reaction pathways, stability and opportunities. J. Mater. Chem. A 2020, 8, 24833–24867.
- Jiang, L.; Dong, M.; Dou, Y.; Chen, S.; Liu, P.; Yin, H.; Zhao, H. Manganese oxides transformed from orthorhombic phase to birnessite with enhanced electrochemical performance as supercapacitor electrodes. J. Mater. Chem. A 2020, 8, 3746–3753.
- Luo, J.; Huang, A.; Park, S.H.; Suib, S.L.; O’Young, C.-L. Crystallization of Sodium-Birnessite and Accompanied Phase Transformation. Chem. Mater. 1998, 10, 1561–1568.
- Chen, S.; Liao, Z.; Kang, J.; Zhang, Y.; Zhi, S.; Cai, X.; Yang, W.; Zou, H.; Yang, W. Enhanced cyclic performance of O2-type Mn-based layered oxide via Al doping for lithium-ion battery. J. Alloys Compd. 2022, 910, 164793.
- Song, T.; Chen, L.; Gastol, D.; Dong, B.; Marco, J.F.; Berry, F.; Slater, P.; Reed, D.; Kendrick, E. High-Voltage Stabilization of O3-Type Layered Oxide for Sodium-Ion Batteries by Simultaneous Tin Dual Modification. Chem. Mater. 2022, 34, 4153–4165.
- Su, D.; Wang, C.; Ahn, H.-J.; Wang, G. Single Crystalline Na0.7MnO2 Nanoplates as Cathode Materials for Sodium-Ion Batteries with Enhanced Performance. Chem. Eur. J. 2013, 19, 10884–10889.
- Shibata, T.; Fukuzumi, Y.; Kobayashi, W.; Moritomo, Y. Fast discharge process of layered cobalt oxides due to high Na+ diffusion. Sci. Rep. 2015, 5, 9006.
- Li, G.; Zhu, W.; Liu, W. First-principles calculations of the Ti-doping effects on layered NaNiO2 cathode materials for advanced Na-ion batteries. J. Indian. Chem. Soc. 2022, 99, 100424.
- Kanwade, A.; Gupta, S.; Kankane, A.; Tiwari, M.K.; Srivastava, A.; Kumar Satrughna, J.A.; Chand Yadav, S.; Shirage, P.M. Transition metal oxides as a cathode for indispensable Na-Ion batteries. RSC Adv. 2022, 12, 23284–23310.
- Heubner, C.; Matthey, B.; Lein, T.; Wolke, F.; Liebmann, T.; Lämmel, C.; Schneider, M.; Herrmann, M.; Michaelis, A. Insights into the electrochemical Li/Na-exchange in layered LiCoO2 cathode material. Energy Stor. Mater. 2020, 27, 377–386.
- Rai, A.K.; Anh, L.T.; Gim, J.; Mathew, V.; Kim, J. Electrochemical properties of NaxCoO2 (x~0.71) cathode for rechargeable sodium-ion batteries. Ceram. Int. 2014, 40, 2411–2417.
- Vassilaras, P.; Ma, X.; Li, X.; Ceder, G. Electrochemical Properties of Monoclinic NaNiO2. J. Electrochem. Soc. 2013, 160, A207.
- Rami Reddy, B.V.; Ravikumar, R.; Nithya, C.; Gopukumar, S. High performance NaxCoO2 as a cathode material for rechargeable sodium batteries. J. Mater. Chem. A 2015, 3, 18059–18063.
- Park, K.; Yu, B.-C.; Goodenough, J.B. Electrochemical and Chemical Properties of Na2NiO2 as a Cathode Additive for a Rechargeable Sodium Battery. Chem. Mater. 2015, 27, 6682–6688.
- Chen, T.; Ouyang, B.; Fan, X.; Zhou, W.; Liu, W.; Liu, K. Oxide cathodes for sodium-ion batteries: Designs, challenges, and perspectives. Carbon. Energy 2022, 4, 170–199.
- Zhang, R.; Lu, Z.; Yang, Y.; Shi, W. First-principles investigation of the monoclinic NaMnO2 cathode material for rechargeable Na-ion batteries. Curr. Appl. Phys. 2018, 18, 1431–1435.
- Palluzzi, M.; Silvestri, L.; Celeste, A.; Tuccillo, M.; Latini, A.; Brutti, S. Structural Degradation of O3-NaMnO2 Positive Electrodes in Sodium-Ion Batteries. Crystals 2022, 12, 885.
- Ma, X.; Chen, H.; Ceder, G. Electrochemical Properties of Monoclinic NaMnO2. J. Electrochem. Soc. 2011, 158, A1307.
- Xiao, J.; Li, X.; Tang, K.; Wang, D.; Long, M.; Gao, H.; Chen, W.; Liu, C.; Liu, H.; Wang, G. Recent progress of emerging cathode materials for sodium ion batteries. Mater. Chem. Front. 2021, 5, 3735–3764.
- Gupta, P.; Pushpakanth, S.; Haider, M.A.; Basu, S. Understanding the Design of Cathode Materials for Na-Ion Batteries. ACS Omega 2022, 7, 5605–5614.
- Kwon, M.-S.; Lim, S.G.; Park, Y.; Lee, S.-M.; Chung, K.Y.; Shin, T.J.; Lee, K.T. P2 Orthorhombic Na0.7O2+y as Cathode Materials for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 14758–14768.
- Nanthagopal, M.; Ho, C.W.; Shaji, N.; Sim, G.S.; Varun Karthik, M.; Kim, H.K.; Lee, C.W. Enhanced NaFe0.5Mn0.5O2/C Nanocomposite as a Cathode for Sodium-Ion Batteries. Nanomaterials 2022, 12, 984.
- Liu, X.; Zuo, W.; Zheng, B.; Xiang, Y.; Zhou, K.; Xiao, Z.; Shan, P.; Shi, J.; Li, Q.; Zhong, G.; et al. P2-Na0.67AlxMn1−xO2: Cost-Effective, Stable and High-Rate Sodium Electrodes by Suppressing Phase Transitions and Enhancing Sodium Cation Mobility. Angew. Chem. Int. Ed. 2019, 58, 18086–18095.
- Abou-Rjeily, J.; Bezza, I.; Laziz, N.A.; Neacsa, D.; Autret-Lambert, C.; Ghamouss, F. P2-Na0.67Mn0.85Al0.15O2 and NaMn2O4 Blend as Cathode Materials for Sodium-Ion Batteries Using a Natural β-MnO2 Precursor. ACS Omega 2021, 6, 1064–1072.
- Shi, Q.; Qi, R.; Feng, X.; Wang, J.; Li, Y.; Yao, Z.; Wang, X.; Li, Q.; Lu, X.; Zhang, J.; et al. Niobium-doped layered cathode material for high-power and low-temperature sodium-ion batteries. Nat. Commun. 2022, 13, 3205.
- Zuo, W.; Liu, X.; Qiu, J.; Zhang, D.; Xiao, Z.; Xie, J.; Ren, F.; Wang, J.; Li, Y.; Ortiz, G.F.; et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries. Nat. Commun. 2021, 12, 4903.
- Clément, R.J.; Bruce, P.G.; Grey, C.P. Review—Manganese-Based P2-Type Transition Metal Oxides as Sodium-Ion Battery Cathode Materials. J. Electrochem. Soc. 2015, 162, A2589.
- Kataoka, R.; Mukai, T.; Yoshizawa, A.; Sakai, T. Development of High Capacity Cathode Material for Sodium Ion Batteries Na0.95Li0.15(Ni0.15Mn0.55Co0.1)O2. J. Electrochem. Soc. 2013, 160, A933–A939.
- Banik, T.; Bhattacharya, I. Novel P2-Type Na0.66Fe0.5-2xMn0.5TixVxO2 Cathode for High-Capacity. In Electrochemical Society Meeting Abstracts 240; MA2021-02; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 238.
- Xu, J.; Lee, D.H.; Clément, R.J.; Yu, X.; Leskes, M.; Pell, A.J.; Pintacuda, G.; Yang, X.-Q.; Grey, C.P.; Meng, Y.S. Identifying the Critical Role of Li Substitution in P2–NaxO2 (0 < x, y, z < 1) Intercalation Cathode Materials for High-Energy Na-Ion Batteries. Chem. Mater. 2014, 26, 1260–1269.
- Fu, F.; Liu, X.; Fu, X.; Chen, H.; Huang, L.; Fan, J.; Le, J.; Wang, Q.; Yang, W.; Ren, Y.; et al. Entropy and crystal-facet modulation of P2-type layered cathodes for long-lasting sodium-based batteries. Nat. Commun. 2022, 13, 2826.
- Yu, C.-Y.; Park, J.-S.; Jung, H.-G.; Chung, K.-Y.; Aurbach, D.; Sun, Y.-K.; Myung, S.-T. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 2015, 8, 2019–2026.
- Yabuuchi, N.; Yoshida, H.; Komaba, S. Crystal Structures and Electrode Performance of Alpha-NaFeO2 for Rechargeable Sodium Batteries. Electrochemistry 2012, 80, 716–719.
- Ono, Y.; Yui, Y.; Hayashi, M.; Asakura, K.; Kitabayashi, H.; Takahashi, K.I. Electrochemical Properties of NaCuO2 for Sodium-Ion Secondary Batteries. ECS Trans. 2014, 58, 33–39.
- Liang, J.; Liu, L.; Liu, X.; Meng, X.; Zeng, L.; Liu, J.; Li, J.; Shi, Z.; Yang, Y. O3-Type NaCrO2 as a Superior Cathode Material for Sodium/Potassium-Ion Batteries Ensured by High Structural Reversibility. ACS Appl. Mater. Interfaces 2021, 13, 22635–22645.
- Myung, S.-T.; Park, J.s.; Jung, H.-G.; Chung, K.Y.; Aurbach, D.; Yu, C.-y.; Sun, Y.-K. NaCrO2 Cathode for High-Rate Sodium-Ionbatteries. In Electrochemical Society Meeting Abstracts 230; MA2016-02; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2016; p. 664.
- Wang, Z.; Shaw, L. Doping of NaCrO2 Cathode Material to Enhance Electrochemical Performance for Sodium-Ion Batteries. In Electrochemical Society Meeting Abstracts 239; MA2021-01; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 356.
- Ono, Y. Structural Analysis of NaCuO2 Cathode at Various Charged/Discharged Stages and Its Reaction Mechanism. Electrochemistry 2018, 86, 309–314.
- Lee, E.; Brown, D.E.; Alp, E.E.; Ren, Y.; Lu, J.; Woo, J.-J.; Johnson, C.S. New Insights into the Performance Degradation of Fe-Based Layered Oxides in Sodium-Ion Batteries: Instability of Fe3+/Fe4+ Redox in α-NaFeO2. Chem. Mater. 2015, 27, 6755–6764.
- Feng, J.; Luo, S.; Cai, K.; Yan, S.; Wang, Q.; Zhang, Y.; Liu, X. Research progress of tunnel-type sodium manganese oxide cathodes for SIBs. Chin. Chem. Lett. 2022, 33, 2316–2326.
- Byles, B.; Pomerantseva, E. Stabilization of Tunnel Manganese Oxide Electrodes in Li-Ion and Na-Ion Batteries. In Electrochemical Society Meeting Abstracts 233; MA2018-01; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2018; p. 2581.
- Wang, Y.; Liu, J.; Lee, B.; Qiao, R.; Yang, Z.; Xu, S.; Yu, X.; Gu, L.; Hu, Y.-S.; Yang, W.; et al. Ti-substituted tunnel-type Na0.44MnO2 oxide as a negative electrode for aqueous sodium-ion batteries. Nat. Commun. 2015, 6, 6401.
- Oz, E.; Altin, S.; Avci, S. Tunnel/Layer Composite Na0.44MnO2 Cathode Material with Enhanced Structural Stability via Cobalt Doping for Sodium-Ion Batteries. ACS Omega 2023, 8, 27170–27178.
- Parant, J.-P.; Olazcuaga, R.; Devalette, M.; Fouassier, C.; Hagenmuller, P. Sur quelques nouvelles phases de formule NaxMnO2 (x ≤ 1). J. Solid. State Chem. 1971, 3, 1–11.
- Hosono, E.; Matsuda, H.; Honma, I.; Fujihara, S.; Ichihara, M.; Zhou, H. Synthesis of single crystalline electro-conductive Na0.44MnO2 nanowires with high aspect ratio for the fast charge–discharge Li ion battery. J. Power Sources 2008, 182, 349–352.
- Zhou, X.; Guduru, R.K.; Mohanty, P. Synthesis and characterization of Na0.44MnO2 from solution precursors. J. Mater. Chem. A 2013, 1, 2757–2761.
- Shen, K.-Y.; Lengyel, M.; Wang, L.; Axelbaum, R.L. Spray pyrolysis and electrochemical performance of Na0.44MnO2 for sodium-ion battery cathodes. MRS Commun. 2017, 7, 74–77.
- Zhang, J.; Yuan, H.; Huang, Y.; Kan, S.; Wu, Y.; Bu, M.; Liu, Y.; He, P.; Liu, H. Engineering sodium-rich manganese oxide with robust tunnel structure for high-performance sodium-ion battery cathode application. Chem. Eng. J. 2021, 417, 128097.
- Chae, M.S.; Elias, Y.; Aurbach, D. Tunnel-Type Sodium Manganese Oxide Cathodes for Sodium-Ion Batteries. ChemElectroChem 2021, 8, 798–811.
- Kim, D.J.; Ponraj, R.; Kannan, A.G.; Lee, H.-W.; Fathi, R.; Ruffo, R.; Mari, C.M.; Kim, D.K. Diffusion behavior of sodium ions in Na0.44MnO2 in aqueous and non-aqueous electrolytes. J. Power Sources 2013, 244, 758–763.
- He, X.; Wang, J.; Qiu, B.; Paillard, E.; Ma, C.; Cao, X.; Liu, H.; Stan, M.C.; Liu, H.; Gallash, T.; et al. Durable high-rate capability Na0.44MnO2 cathode material for sodium-ion batteries. Nano Energy 2016, 27, 602–610.
- Guo, S.; Yu, H.; Liu, D.; Tian, W.; Liu, X.; Hanada, N.; Ishida, M.; Zhou, H. A novel tunnel Na0.61Ti0.48Mn0.52O2 cathode material for sodium-ion batteries. Chem. Commun. 2014, 50, 7998–8001.
- Xu, S.; Wang, Y.; Ben, L.; Lyu, Y.; Song, N.; Yang, Z.; Li, Y.; Mu, L.; Yang, H.-T.; Gu, L.; et al. Fe-Based Tunnel-Type Na0.61O2 Designed by a New Strategy as a Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2015, 5, 1501156.
- Tang, W.; Song, X.; Du, Y.; Peng, C.; Lin, M.; Xi, S.; Tian, B.; Zheng, J.; Wu, Y.; Pan, F.; et al. High-performance NaFePO4 formed by aqueous ion-exchange and its mechanism for advanced sodium ion batteries. J. Mater. Chem. A 2016, 4, 4882–4892.
- Ling, M.; Lv, Z.; Li, F.; Zhao, J.; Zhang, H.; Hou, G.; Zheng, Q.; Li, X. Revisiting of Tetragonal NaVPO4F: A High Energy Density Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 30510–30519.
- Gutierrez, A.; Kim, S.; Fister, T.T.; Johnson, C.S. Microwave-Assisted Synthesis of NaCoPO4 Red-Phase and Initial Characterization as High Voltage Cathode for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4391–4396.
- Priyanka, V.; Savithiri, G.; Subadevi, R.; Sivakumar, M. An emerging electrochemically active maricite NaMnPO4 as cathode material at elevated temperature for sodium-ion batteries. Appl. Nanosci. 2020, 10, 3945–3951.
- Mathew, V.; Kim, S.; Kang, J.; Gim, J.; Song, J.; Baboo, J.P.; Park, W.; Ahn, D.; Han, J.; Gu, L.; et al. Amorphous iron phosphate: Potential host for various charge carrier ions. NPG Asia Mater. 2014, 6, e138.
- Wang, R.; Wu, S.; Zhang, F.; Zhao, X.; Lin, Z.; Wang, C.-Z.; Ho, K.-M. Stabilizing the crystal structures of NaFePO4 with Li substitutions. Phys. Chem. Chem. Phys. 2020, 22, 13975–13980.
- Jian, Z.; Yuan, C.; Han, W.; Lu, X.; Gu, L.; Xi, X.; Hu, Y.-S.; Li, H.; Chen, W.; Chen, D.; et al. Atomic Structure and Kinetics of NASICON NaxV2(PO4)3 Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2014, 24, 4265–4272.
- Goodenough, J.B.; Hong, H.Y.P.; Kafalas, J.A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 1976, 11, 203–220.
- Hong, H.Y.P. Crystal structures and crystal chemistry in the system Na1+xZr2SixP3−xO12. Mater. Res. Bull. 1976, 11, 173–182.
- Oh, J.A.S.; He, L.; Plewa, A.; Morita, M.; Zhao, Y.; Sakamoto, T.; Song, X.; Zhai, W.; Zeng, K.; Lu, L. Composite NASICON (Na3Zr2Si2PO12) Solid-State Electrolyte with Enhanced Na+ Ionic Conductivity: Effect of Liquid Phase Sintering. ACS Appl. Mater. Interfaces 2019, 11, 40125–40133.
- Gopalakrishnan, J.; Rangan, K.K. Vanadium phosphate (V2(PO4)3): A novel NASICO N-type vanadium phosphate synthesized by oxidative deintercalation of sodium from sodium vanadium phosphate (Na3V2(PO4)3). Chem. Mater. 1992, 4, 745–747.
- Zhu, Y.; Xu, H.; Ma, J.; Chen, P.; Chen, Y. The recent advances of NASICON-Na3V2(PO4)3 cathode materials for sodium-ion batteries. J. Solid. State Chem. 2023, 317, 123669.
- Pandit, B.; Sougrati, M.T.; Fraisse, B.; Monconduit, L. Exploration of a Na3V2(PO4)3/C-Pb full cell Na-ion prototype. Nano Energy 2022, 95, 107010.
- Zhang, X.; Rui, X.; Chen, D.; Tan, H.; Yang, D.; Huang, S.; Yu, Y. Na3V2(PO4)3: An advanced cathode for sodium-ion batteries. Nanoscale 2019, 11, 2556–2576.
- Wei, P.; Chen, W.; Jing, Q.; Lee, M.-H.; Chen, Z. Effects of P2O7 clusters arrangement on second harmonic generation responses of pyrophosphates. J. Alloys Compd. 2020, 827, 153922.
- Niu, Y.; Zhang, Y.; Xu, M. A review on pyrophosphate framework cathode materials for sodium-ion batteries. J. Mater. Chem. A 2019, 7, 15006–15025.
- Uebou, Y.; Okada, S.; Yamaki, J.-I. Electrochemical insertion of lithium and sodium into (MoO2)2P2O7. J. Power Sources 2003, 115, 119–124.
- Gabelica-Robert, M.; Goreaud, M.; Labbe, P.; Raveau, B. The pyrophosphate NaFeP2O7: A cage structure. J. Solid. State Chem. 1982, 45, 389–395.
- Barpanda, P.; Lu, J.; Ye, T.; Kajiyama, M.; Chung, S.-C.; Yabuuchi, N.; Komaba, S.; Yamada, A. A layer-structured Na2CoP2O7 pyrophosphate cathode for sodium-ion batteries. RSC Adv. 2013, 3, 3857–3860.
- Barpanda, P.; Ye, T.; Avdeev, M.; Chung, S.-C.; Yamada, A. A new polymorph of Na2MnP2O7 as a 3.6 V cathode material for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 4194–4197.
- Barpanda, P.; Liu, G.; Ling, C.D.; Tamaru, M.; Avdeev, M.; Chung, S.-C.; Yamada, Y.; Yamada, A. Na2FeP2O7: A Safe Cathode for Rechargeable Sodium-ion Batteries. Chem. Mater. 2013, 25, 3480–3487.
- Kim, H.; Park, C.S.; Choi, J.W.; Jung, Y. Defect-Controlled Formation of Triclinic Na2CoP2O7 for 4 V Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 6662–6666.
- Ha, K.-H.; Woo, S.H.; Mok, D.; Choi, N.-S.; Park, Y.; Oh, S.M.; Kim, Y.; Kim, J.; Lee, J.; Nazar, L.F.; et al. Na4−αM2+α/2(P2O7)2 (2/3 ≤ α ≤ 7/8, M = Fe, Fe0.5Mn0.5, Mn): A Promising Sodium Ion Cathode for Na-ion Batteries. Adv. Energy Mater. 2013, 3, 770–776.
- Erragh, F.; Boukhari, A.; Abraham, F.; Elouadi, B. Study of the Crystal Structures of Sodium Magnesium and Sodium Nickel Diphosphates. J. Solid. State Chem. 2000, 152, 323–331.
- Liu, G.; Nishimura, S.-I.; Chung, S.C.; Fujii, K.; Yashima, M.; Yamada, A. Defect induced sodium disorder and ionic conduction mechanism in Na1.82Mg1.09P2O7. J. Mater. Chem. A 2014, 2, 18353–18359.
- Kim, H.; Shakoor, R.A.; Park, C.; Lim, S.Y.; Kim, J.-S.; Jo, Y.N.; Cho, W.; Miyasaka, K.; Kahraman, R.; Jung, Y.; et al. Na2FeP2O7 as a Promising Iron-Based Pyrophosphate Cathode for Sodium Rechargeable Batteries: A Combined Experimental and Theoretical Study. Adv. Funct. Mater. 2013, 23, 1147–1155.
- Masese, T.; Orikasa, Y.; Tassel, C.; Kim, J.; Minato, T.; Arai, H.; Mori, T.; Yamamoto, K.; Kobayashi, Y.; Kageyama, H.; et al. Relationship between Phase Transition Involving Cationic Exchange and Charge-Discharge Rate in Li2FeSiO4. Chem. Mater. 2014, 26, 1380–1384.
- Gao, S.; Zhao, J.; Zhao, Y.; Wu, Y.; Zhang, X.; Wang, L.; Liu, X.; Rui, Y.; Xu, J. Na2CoSiO4 as a novel positive electrode material for sodium-ion capacitors. Mater. Lett. 2015, 158, 300–303.
- Treacher, J.C.; Wood, S.M.; Islam, M.S.; Kendrick, E. Na2CoSiO4 as a cathode material for sodium-ion batteries: Structure, electrochemistry and diffusion pathways. Phys. Chem. Chem. Phys. 2016, 18, 32744–32752.
- Wang, J.; Hoteling, G.; Shepard, R.; Wahila, M.; Wang, F.; Smeu, M.; Liu, H. Reaction Mechanism of Na-Ion Deintercalation in Na2CoSiO4. J. Phys. Chem. C 2022, 126, 16983–16992.
- Pedone, A.; Malavasi, G.; Menziani, M.C.; Cormack, A.N.; Segre, U. A New Self-Consistent Empirical Interatomic Potential Model for Oxides, Silicates, and Silica-Based Glasses. J. Phys. Chem. B 2006, 110, 11780–11795.
- Wu, P.; Wu, S.Q.; Lv, X.; Zhao, X.; Ye, Z.; Lin, Z.; Wang, C.Z.; Ho, K.M. Fe–Si networks in Na2FeSiO4 cathode materials. Phys. Chem. Chem. Phys. 2016, 18, 23916–23922.
- Bianchini, F.; Fjellvåg, H.; Vajeeston, P. First-principles study of the structural stability and electrochemical properties of Na2MSiO4 (M = Mn, Fe, Co and Ni) polymorphs. Phys. Chem. Chem. Phys. 2017, 19, 14462–14470.
- Jin, T.; Li, H.; Zhu, K.; Wang, P.-F.; Liu, P.; Jiao, L. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377.
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 2016, 45, 6345–6404.
- Wang, H.-G.; Yuan, S.; Ma, D.-L.; Huang, X.-L.; Meng, F.-L.; Zhang, X.-B. Tailored Aromatic Carbonyl Derivative Polyimides for High-Power and Long-Cycle Sodium-Organic Batteries. Adv. Energy Mater. 2014, 4, 1301651.
- Wang, S.; Wang, L.; Zhu, Z.; Hu, Z.; Zhao, Q.; Chen, J. All Organic Sodium-Ion Batteries with Na4C8H2O6. Angew. Chem. 2014, 126, 6002–6006.
- Wang, D.-Y.; Liu, R.; Guo, W.; Li, G.; Fu, Y. Recent advances of organometallic complexes for rechargeable batteries. Coord. Chem. Rev. 2021, 429, 213650.
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259.
- Li, X.; Yang, X.; Xue, H.; Pang, H.; Xu, Q. Metal–organic frameworks as a platform for clean energy applications. EnergyChem 2020, 2, 100027.
- Du, M.; Li, Q.; Zhao, Y.; Liu, C.-S.; Pang, H. A review of electrochemical energy storage behaviors based on pristine metal–organic frameworks and their composites. Coord. Chem. Rev. 2020, 416, 213341.
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546.
- Yi, H.; Qin, R.; Ding, S.; Wang, Y.; Li, S.; Zhao, Q.; Pan, F. Structure and Properties of Prussian Blue Analogues in Energy Storage and Conversion Applications. Adv. Funct. Mater. 2020, 31, 2006970.
- Luo, Y.; Peng, J.; Yin, S.; Xue, L.; Yan, Y. Acid-Assisted Ball Mill Synthesis of Carboxyl-Functional-Group-Modified Prussian Blue as Sodium-Ion Battery Cathode. Nanomaterials 2022, 12, 1290.
- Xie, B.X.; Sun, B.Y.; Gao, T.Y.; Ma, Y.L.; Yin, G.P.; Zuo, P.J. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478.
- Fu, H.; Liu, C.; Zhang, C.; Ma, W.; Wang, K.; Li, Z.; Lu, X.; Cao, G. Enhanced storage of sodium ions in Prussian blue cathode material through nickel doping. J. Mater. Chem. A 2017, 5, 9604–9610.
- Qian, J.F.; Zhou, M.; Cao, Y.L.; Ai, X.P.; Yang, H.X. Nanosized Na4Fe(CN)6/C Composite as a Low-Cost and High-Rate Cathode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2012, 2, 410–414.
- Li, W.-J.; Chou, S.-L.; Wang, J.-Z.; Kang, Y.-M.; Wang, J.-L.; Liu, Y.; Gu, Q.-F.; Liu, H.-K.; Dou, S.-X. Facile Method To Synthesize Na-Enriched Na1+xFeFe(CN)6 Frameworks as Cathode with Superior Electrochemical Performance for Sodium-Ion Batteries. Chem. Mater. 2015, 27, 1997–2003.
- Yang, D.; Liao, X.-Z.; Huang, B.; Shen, J.; He, Y.-S.; Ma, Z.-F. A Na4Fe(CN)6/NaCl solid solution cathode material with an enhanced electrochemical performance for sodium ion batteries. J. Mater. Chem. A 2013, 1, 13417–13421.
- Yu, T.-Y.; Sun, Y.-K. A fluorinated O3-type layered cathode for long-life sodium-ion batteries. J. Mater. Chem. A 2022, 10, 23639–23648.
- Zhou, Q.; Wang, L.; Li, W.; Zhao, K.; Liu, M.; Wu, Q.; Yang, Y.; He, G.; Parkin, I.P.; Shearing, P.R.; et al. Sodium Superionic Conductors (NASICONs) as Cathode Materials for Sodium-Ion Batteries. Electrochem. Energy Rev. 2021, 4, 793–823.
- Wang, Q.; Wu, X.; You, H.; Min, H.; Xu, X.; Hao, J.; Liu, X.; Yang, H. Template-directed Prussian blue nanocubes supported on Ni foam as the binder-free anode of lithium-ion batteries. Appl. Surf. Sci. 2022, 571, 151194.
- Kee, Y.; Dimov, N.; Staykov, A.; Okada, S. Investigation of metastable Na2FeSiO4 as a cathode material for Na-ion secondary battery. Mater. Chem. Phys. 2016, 171, 45–49.
- Kim, H.; Sadan, M.K.; Kim, C.; Jo, J.; Seong, M.; Cho, K.-K.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Enhanced reversible capacity of sulfurized polyacrylonitrile cathode for room-temperature Na/S batteries by electrochemical activation. Chem. Eng. J. 2021, 426, 130787.
- Huang, Z.-X.; Zhang, X.-L.; Zhao, X.-X.; Zhao, Y.-Y.; Aravindan, V.; Liu, Y.-H.; Geng, H.; Wu, X.-L. Electrode/electrolyte additives for practical sodium-ion batteries: A mini review. Inorg. Chem. Front. 2023, 10, 37–48.
- Hou, Y.; Jin, J.; Huo, C.; Liu, Y.; Deng, S.; Chen, J. New insights into the critical role of inactive element substitution in improving the rate performance of sodium oxide cathode material. Energy Stor. Mater. 2023, 56, 87–95.





