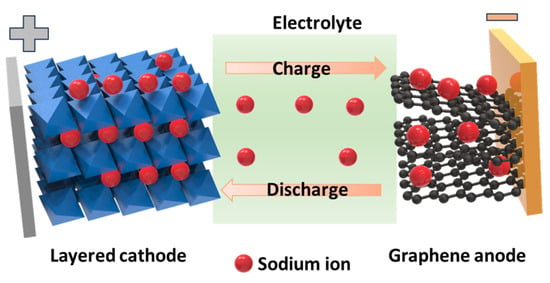
Emerging energy storage systems have received significant attention along with the development of renewable energy, thereby creating a green energy platform for humans. Lithium-ion batteries (LIBs) are commonly used, such as in smartphones, tablets, earphones, and electric vehicles. However, lithium has certain limitations including safety, cost-effectiveness, and environmental issues. Sodium is believed to be an ideal replacement for lithium owing to its infinite abundance, safety, low cost, environmental friendliness, and energy storage behavior similar to that of lithium. Inhered in the achievement in the development of LIBs, sodium-ion batteries (SIBs) have rapidly evolved to be commercialized. Among the cathode, anode, and electrolyte, the cathode remains a significant challenge for achieving a stable, high-rate, and high-capacity device.
- sodium-ion batteries
- cathode materials
- inorganic cathodes
- organic cathodes
1. Introduction
2. SIB Cathode Materials
2.1. Inorganic Compounds
2.1.1. Layered Oxide Materials (Na
x
MO
2
)
2.1.2. Tunnel Oxides
The Na2.1.3. Polyanionic Compounds
Phosphate-Based Compound
NASICON
A Na super-ionic conductor (NASICON) can be used as an electrolyte and electrode material owing to its 3D-open framework of NaxM2(PO4)3 (M = V, Fe, Ti, Nb, Zr) [97][115]. NASICON comprises MO6 and PO4 polyhedral sites in a framework that creates large channels for Na diffusion. This structure was first proposed by Hong and Goodenough in a Na1+xZr2P3-xSixO12 compound (P can be replaced by Si, S, Mo, and As) [98][99][116,117]. Owing to its high stability, high Na conductivity, and wide electrochemical windows (1.85–4.9 V vs. Na/Na+), NASICON is also applied as a solid electrolyte in SIBs [100][118]. The ion exchange of Zr4+ with Li+, K+, and Ag+ was first performed, while Si4+ was stabilized in the structure. As a complete NASICON with three full Na ions, Na3V2(PO4)3 (NVP) quickly received significant attention as a promising candidate material for providing a high probability of sodium insertion and desertion [101][102][119,120]. NVP has a theoretical capacity of ~117.6 mAh g−1 and a high redox voltage range of 3.3–3.4 V [103][121]. Therefore, with the modification process including the addition of conductive carbonaceous materials, NVP conductivity can be enhanced, exhibiting a notable rate performance [104][122].2.1.4. Pyrophosphates
Pyrophosphate NaxMP2O7 consists of MO6 (M = V, Fe, Mn, Co, Ni) sites and a P2O7 group (interconnected PO4–PO4) that forms a framework with Na ions [105][106][107][108][109][110][130,131,132,133,134,135]. This framework allows the diffusion of Na ions; therefore, it is also a stable cathode material for SIBs. Barpanda et al. revealed that Na2FeP2O7 was constructed by corner-sharing FeO6–FeO6 to form Fe2O11, which combines with the P2O7 group to form a triclinic structure [111][136]. After calcination at temperatures above 560 °C, the triclinic Na2PeP2O7 transformed into a monoclinic phase, which improved the stability of this material during cycling. Kim et al. used the defect engineering of Na in Na2CoP2O7 to produce a high-voltage cathode for SIBs [112][137]. The deficiency of the Na-stabilized structure of Na2-xCoP2O7 (x > 0.2) was also found in Fe, Ni, and Mg pyrophosphates, such as Na1.66Fe1.17P2O7, Na1.82Ni1.09P2O7, and Na1.82Mg1.09P2O7 [113][114][115][116][138,139,140,141]. Specifically, Na2-xCo2P2O7 (x > 0.2) achieved a high average voltage of approximately 4.3 V versus Na/Na+ with a specific capacity of approximately 80 mAh g−1. Owing to the similar roles of the V, Fe, Mn, Co, and Ni transition metals in the structure, the replacement of a cheaper metal such as Fe and the improvement of the voltage by using Co and Ni in other pyrophosphate materials were investigated.2.1.5. Silicates
Silicate compounds, such as lithium orthosilicate Li2FeSiO4 with a theoretical capacity of approximately 300 mAh g−1, generally have a higher theoretical capacity than other polyanions owing to their low molecular weight [117][146]. Similar to Li2FeSiO4, the sodium silicate Na2MSiO4 compound consists of MO4 (M = Fe, Ni, Mn, Co) and SiO4 sites, forming a framework that allows the diffusion of Na ions [118][119][120][147,148,149]. Silicates were previously popular in the glass industry owing to their high thermal and physical stabilities [121][150]. Co/Fe-compound sodium silicates were predicted to exhibit anti-site-exchange behavior, promising to be stable electrode materials [122][123][151,152]. Na2FeSiO4 is the most promising silicate compound, having a high theoretical capacity of approximately 276 mAh g−1 [124][153].2.2. Organic Compounds
The development of flexible devices and environmentally friendly materials has encouraged the application of organic compounds as cathode materials in energy storage systems, such as LIBs and SIBs [125][158]. Ranging from small molecules to high-molecular polymers, organic materials are promising for applications in green renewable energy in the future. For example, the molecular structure of Na4C8H2O6 (2,5-dihydroxyterephthalic acid, NaDTA) was investigated as a SIB cathode material at working potential windows of approximately 1.6–2.8 V versus Na/Na+ and delivered a high capacity of approximately 180 mAh g−1 [126][159]. NaDTA can also be used as an anode material with a capacity greater than 200 mAh g−1 owing to it binding up to six Na ions [127][160]. Kim et al. demonstrated the use of C6Cl4O2 (tetrachloro-1,4-benzoquinone) in a porous carbon template as a cathode of SIBs. The carbon skeleton-supported C6Cl4O2 cathode exhibited a high initial capacity of approximately 160 mAh g−1 and an average voltage of approximately 2.72 V. Wang et al. produced a polymer from perylene 3,4,9,10-tetracarboxylic dianhydride, pyromellitic dianhydride (PMDA), and 1,4,5,8-naphthalenetetracarboxylic dianhydride, which contained C=O bindings, providing interactions with Na+ ions as a cathode for SIBs. This polymer demonstrated a high reversible capacity of approximately 150 mAh g−1 at a working voltage of 1.5–3.5 V and a long lifetime of over 5000 cycles, retaining 87.5% of the capacity in comparison to the initial cycle.2.3. Metal–Organic Compounds: Prussian Blue Analogs
The combination of inorganic and organic structures has received considerable attention owing to the advantages of both material types [128][166]. Inorganic materials have a stable structure and high conductivity, whereas organic materials are eco-friendly, easy to process, and safe to use. Recently, the development of organometallic materials in framework structures has introduced an advanced technique for material design, enabling the discovery of new composite properties for metals and organics. Metal–organic frameworks (MOF) can form a tremendous structure from various metal–organic compounds, providing large channels that allow the capture of ions or molecules; therefore, they have been used in various applications, including drug delivery, catalysis, and energy storage [129][130][131][167,168,169]. Simple and famous MOFs used for energy storage are Prussian blue analogs (PBAs), which are alkaline metal ferrocyanides AxMFe(CN)6 (A = Na, K; M = Fe, Mn, Co, Ni, Cu) [132][170]. The CN, Fe, and M matrices create a cage-like structure, holding the Na and K ions. PBAs generally exhibit a face-centered cubic structure (Fm3-m) [133][134][135][171,172,173]. The performance of PBAs in SIBs is based on the redox reactions of Fe2+/Fe3+ and the metal M, believed to have a high theoretical capacity of approximately 170 mAh g−1 for SIBs [136][174]. The basic PBA, which is Na4Fe(CN)6, contains the highest number of Na ions; however, it is a soluble compound that is easily degraded during cycling [137][138][175,176]. Therefore, Yang et al. demonstrated a solid solution of Na4Fe(CN)6/NaCl in a SIB that exhibited a capacity of approximately 75 mAh g−1 [139][177].3. Summary
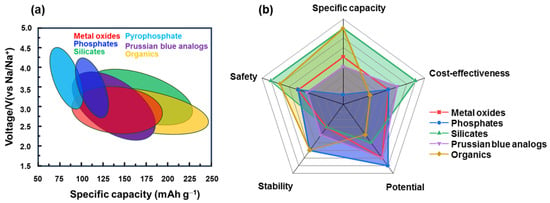
LIBs have become popular in portable devices, vehicles, and energy storage systems for renewable energy. Owing to the abundance of Na, SIBs are believed to be an ideal replacement for LIBs. As shown in Figure 29, each type of cathode material has its advantages and disadvantages. For instance, layered metal oxides have a high capacity and low cost but are sensitive to moisture and structural degradation. Prussian blue is more stable but the effect of water molecules in the structure affects its performance. Organic cathode materials have a good flexibility and stable redox potential but their lower conductivity, thermal stability, and dissolvability in the electrolyte should be resolved. Therefore, the advantages and disadvantages of each practical condition should be carefully considered. To improve their performance, the approach methods were also varied for each type of material. Due to an instability in structure of layered metal oxide cathodes, they were fast degraded during cycling. To stabilize structural stability, inactive metals such as V, Mg, Zn, and Ca can be doped to the lattice, or anions like F can be added [140][141][197,198]. Considering a tunnel metal oxide, control of the tunnel size optimizes its capacity. Meanwhile, for polyanionic compounds such as NASICON or other phosphate-based compounds, defect engineering can be considered, including metal- and F-doping methods [142][199]. Silicate compounds are low-cost and eco-friendly metal sources, and their high capacity needs to improve the structural stability before commercialization [143][154]. The surfaces of inorganic compounds can be passivated using a carbon-coating method that not only enhances their conductivity but also protects against the effects of humidity or expansion during the insertion of Na ions. The stability of Prussian blue and other organometallic compounds can be enhanced by using a host material such as Ni foam or a porous carbon skeleton [144][200]. Organic materials can be designed to have a good structure to enhance capacity and conductivity but they remain in the activation group with C=O, C=C, or C=N. Sulfurization and other cross-linking methods can also be considered to yield better combinations [145][201]. In addition, the use of additives in the electrolyte is another approach to enhance stability, in which the solid electrolyte interface from cycling can be used as a protective layer [146][202]. Along with the development of electrode materials and electrolytes, SIBs have been commercialized with layered oxides, polyanions, and Prussian blue types [32]. These materials are simple to manufacture (hydrothermal, co-precipitation method, etc.) and inexpensive, and they mainly use Mn and Fe metals and add Ni, Zn, or Mg, to increase stability, and conductive carbon is introduced for air stability and structural protection. Organic materials with low thermal stability and conductivity are utilized for some specific purposes that require biocompatible and/or specified applications. Therefore, it is considered that most of the developed materials have the potential to be commercialized if SIBs can solve current issues such as cost-effectiveness, high capacity, high stability, and high rate performance.