
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peng Xue | -- | 107 | 2023-09-04 15:19:41 | | | |
| 2 | Peng Xue | -70 word(s) | 37 | 2023-09-04 16:22:58 | | | | |
| 3 | Peng Xue | + 1592 word(s) | 1629 | 2023-09-05 05:33:07 | | | | |
| 4 | Lindsay Dong | -2 word(s) | 1627 | 2023-09-08 10:46:03 | | | | |
| 5 | Lindsay Dong | Meta information modification | 1627 | 2023-10-24 02:04:47 | | |
Video Upload Options
Cryptococcus neoformans is a major cause of fungal meningitis in immunocompromised individuals. Similar to other melanized microorganisms associated with human diseases, the cell wall-associated melanin of C. neoformans is a major virulence factor enabling it to evade host immune responses. The levels and formation of these melanins may be influenced by the microbiota-gut-brain axis. Studies have also found that C. neoformans infection can lead to dysbiosis of the human gut microbiota.
1. Introductions
Cryptococcus neoformans is a human pathogenic fungus belonging to the phylum Basidiomycota [1][2]. It has a well-defined bipolar mating system, consisting of two different mating types, MATa and MATα [3][4], but is also capable of self-fertilization within cells of the same mating type [5][6]. C. neoformans is widely distributed in the environment, particularly in bird droppings, soil, and trees [7]. It poses a particular danger to immunocompromised hosts, such as HIV/AIDS patients and organ transplant recipients, as it can easily cause meningitis [8][9][10][11][12][13]. For instance, C. neoformans is estimated to cause approximately 223,100 new cases and 181,100 deaths annually among HIV/AIDS patients [14][15]. According to the classification by the World Health Organization (WHO), these species are classified as one of the four priority fungal pathogens affecting humans. Cryptococcus neoformans infection typically occurs through inhalation of fungal spores or desiccated yeast cells in the environment [16][17], but can also occur through skin wounds [18]. These species are able to overcome host defenses and enter the bloodstream, subsequently invading the central nervous system and causing meningitis [16][19]. The diagnosis of cryptococcal meningitis typically involves a comprehensive evaluation of clinical symptoms and signs, combined with various diagnostic methods [20][21][22][23]. These methods include assessing the patient's medical history and performing physical examinations to identify symptoms associated with cryptococcal meningitis. Lumbar puncture is a routine procedure used to obtain and analyze cerebrospinal fluid (CSF) to determine the presence of Cryptococcus species. Laboratory tests include India ink staining of the CSF, culture of Cryptococcus species, or antigen testing to confirm the presence of Cryptococcus infection. Neuroimaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) can be used to examine the brain for signs of meningitis or other abnormalities. The combined application of these methods is crucial for accurate diagnosis and exclusion of other potential causes. It has been suggested that the composition of the gut microbiota may play a potential role in the diagnosis of cryptococcal meningitis. Studies have shown that alterations in the gut microbiota may be associated with fungal infections, including Cryptococcus species [24]. C. neoformans is able to impair the accurate recognition of fungal antigens by the host and evade host immune responses, including the coordinated action of macrophages, dendritic cells, T lymphocytes, B lymphocytes, innate lymphoid cells, and cytokines [25][26]. Furthermore, C. neoformans utilizes melanin production to evade host immunity and enhance its infectivity in the host. Melanin in the cell wall of C. neoformans is a major virulence factor with multiple functions, such as resistance to oxidative stress, reduction of antifungal drug efficacy, and modulation of interactions with phagocytic cells [16][27][28]. Researchers believe that melanin may be a potential target for the development of drugs against Cryptococcus infections [29][30]. A notable feature of C. neoformans is its inability to produce melanin pigments from endogenously synthesized compounds, such as tyrosine, but instead utilizes catecholamines, such as dopamine, norepinephrine, and epinephrine, to oxidize and generate melanin in the brain tissue through the action of laccases (Lac1 and Lac2) [31][32][33]. The neurotropism of C. neoformans is closely related to the presence of catecholamines in the brain and low glucose concentrations in the central nervous system, as these conditions favor laccase production [32][34][35].
2. Cryptococcus neoformans Infection and Dysbiosis of the Gut Microbiota
The latest advances in our understanding and analysis of the gut microbiota have revealed significant impacts of alterations on human health. The gut microbiota is a complex and diverse microbial ecosystem that plays crucial roles in various aspects of host physiology. It actively participates in host immune responses, influences metabolism, promotes biosynthesis, and defends against pathogenic yeast infections [36][37][38][39]. In this regard, it has been found that the composition of the gut bacterial microbiota can influence the production of pulmonary IL-17 responses during opportunistic human fungal pathogen Aspergillus fumigatus infection in mice. Mice with specific gut bacterial compositions exhibited stronger IL-17 responses, which are critical for combating fungal infections [40]. Recently, Li and colleagues reported that C. neoformans infection induces changes in the human gut microbiota [24]. The authors performed alpha and beta diversity analyses, comparing the gut microbiota of cryptococcal meningitis patients with healthy controls. The results showed a significant reduction in alpha diversity in cryptococcal meningitis patients compared to the healthy control group, indicating gut dysbiosis. They identified a total of 72 differentially abundant bacterial species and 8 differentially abundant fungal species between these two groups (Figure 1).
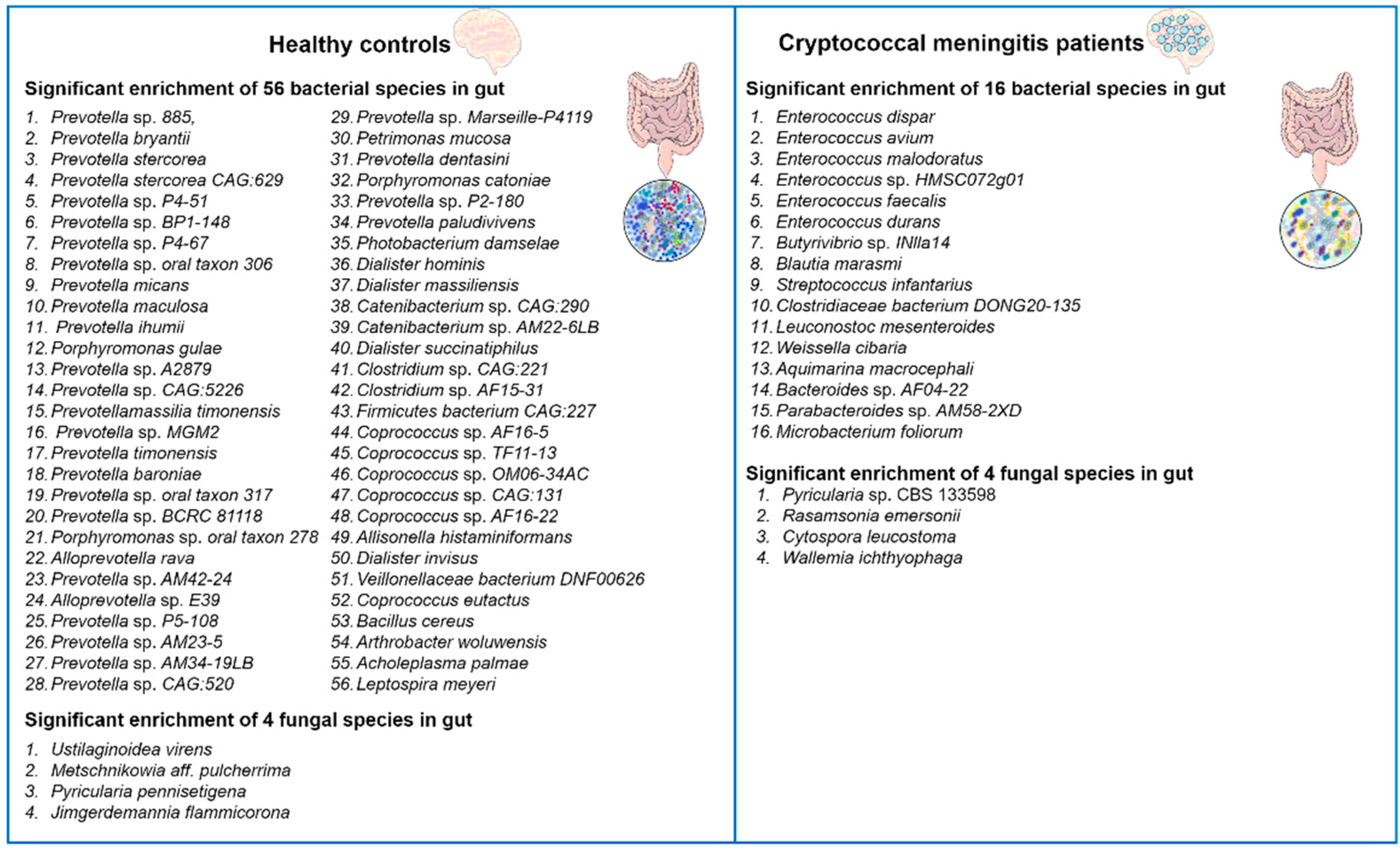
Figure 1. Changes in the diversity of gut bacterial and fungal microbiota in patients with cryptococcal meningitis. There are notable differences in the composition of gut bacterial and fungal microbiota between patients with cryptococcal meningitis and healthy controls. A total of 72 bacterial species and 8 fungal species were found to have differential abundance between these two groups [24].
2. Melanization phenomenon of Cryptococcus neoformans in human brain tissue
The deposition of melanin on the surface of Cryptococcus neoformans cells is one of its important features, and this deposition contributes to its pathogenicity [41][42]. The deposition of melanin depends on the composition and flexibility of the cell wall of C. neoformans [43]. The deposition of melanin within the cell wall provides several advantages for the pathogen [44]. Firstly, melanin acts as a protective barrier and can resist host immune reactions, including phagocytosis by immune cells. Studies have shown that melanin can inhibit the production of reactive oxygen species and reduce the activity of antifungal drugs, thereby enhancing C. neoforman resistance to host defenses. In addition, melanin is associated with the dissemination of C. neoformans within the host. Melanized fungal cells can be detected in various organs, including the brain and lungs, indicating the important role of melanization in the invasion and establishment process in different infected tissues [45]. The main sign of melanization during C. neoformans infection is the presence of acid-resistant melanin ghost particles. These particles can be isolated from infected animal and human tissues, as well as from cells cultured on agar plates [46][47]. The mammalian nervous system is a rich source of catecholamines, which are a class of nitrogen-containing diphenolic compounds, including dopamine, adrenaline, and noradrenaline, among others [48][49]. C. neoformans synthesizes melanin by using the oxidation process of exogenous catecholamine substrates catalyzed by laccase [50][51][52]. Melanized Cryptococcus neoformans cells can be detected in brain tissue samples from patients with cryptococcal meningitis [60]. The melanin synthesized by Cryptococcus neoformans in brain tissue may vary in different anatomical regions, as it can bind to multiple catecholamines simultaneously (see Figure 2). This is because the relative proportions of these neurotransmitters may vary greatly in different brain regions [48][49]. The chemical structure determines the variability of the types of melanin synthesized when added to the culture medium. It is worth noting that Baker et al. made important observations on the ability of Cryptococcus neoformans to produce different forms of melanin using a mixture of catecholamines in the human brain (0.6 mM dopamine, 0.33 mM adrenaline, and 0.07 mM noradrenaline). This melanin can provide protection against UV radiation and oxidants.

Figure 2. Melanization of C. neoformans in different brain tissues. The composition of melanin produced during the infection process may vary depending on the composition of catecholamines in the brain tissue. It is likely that melanin synthesis in vivo is generated through the polymerization of various precursor compounds [27]. Distribution of catecholamines in the brain: the red region represents dopamine enrichment, the green region represents norepinephrine enrichment, and the yellow region represents the overlap of dopamine and norepinephrine. In the cell wall of C. neoformans, the melanin produced in different brain regions can be visualized as red, green, and yellow.
4. Potential impact of the gut microbiota on melanization of Cryptococcus neoformans
4.1. The gut microbiota may influence the levels of melanin substrates
The Gut-brain axis is a bidirectional communication system connecting the gut microbiota and the central nervous system [53][54]. It is generally believed that the gut microbiota has the ability to influence various aspects of brain function, such as emotions, behavior, and cognition [55][56]. This influence may occur through different pathways, such as affecting the production of neurotransmitters and other signaling molecules that can impact the function of the central nervous system [70, 72]. While there is no direct evidence linking the gut-brain axis to the synthesis of melanin in C. neoformans, there is evidence suggesting that the gut microbiota may influence the levels of catecholamines (such as dopamine and norepinephrine) (see Figure 3), which are precursors for melanin synthesis in C. neoformans.
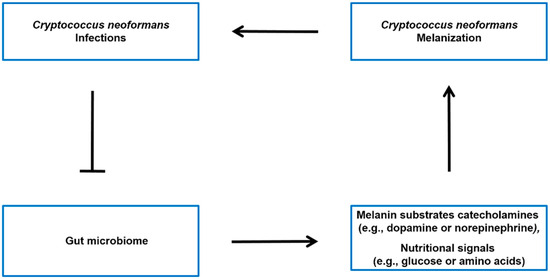
Figure 3. Potential model of the interplay between C. neoformans infection, gut microbiota, and host molecules. It has been observed that there is an association between C. neoformans infection and alterations in the gut microbiota [24]. The microbiota-gut-brain axis may regulate the levels of melanin sunstrate catecholamines (such as dopamine or norepinephrine) [57][58][59], as well as modulate the nutritional signals (such as glucose or amino acids) that influence Cryptococcus neoformans melanin production in the brain [60].
4.2. The gut microbiota may influence the generation of Cryptococcus neoformans melanin by modulating nutritional signals
The ability to rapidly adapt to fluctuating external conditions is crucial for the survival and proliferation of microorganisms. This is especially important for pathogenic microorganisms like C. neoformans, as they need to adapt to the transition from the environment to the host and initiate appropriate responses to establish infection. The host environment presents various challenging conditions, such as changes in available nutrients, oxygen levels, pH, and temperature, as well as potential threats from the host immune response [61][62]. It is noteworthy that the mechanisms for adapting to nutrient availability not only contribute to microbial proliferation but also play a role in regulating their virulence [63].
References
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harb. Perspect. Med. 2014, 4, a019760.
- Zhao, Y.; Lin, X. Cryptococcus neoformans: Sex, morphogenesis, and virulence. Infect. Genet. Evol. 2021, 89, 104731.
- Kwon-Chung, K.J. A new species of Filobasidiella, the sexual state of Cryptococcus neoformans B and C serotypes. Mycologia 1976, 68, 943–946.
- Kwon-Chung, K.J. A new genus, filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 1975, 67, 1197–1200.
- Sun, S.; Heitman, J. From Two to One: Unipolar Sexual Reproduction. Fungal Biol. Rev. 2015, 29, 118–125.
- Wang, L.; Lin, X. Mechanisms of unisexual mating in Cryptococcus neoformans. Fungal Genet. Biol. 2011, 48, 651–660.
- Lin, X.; Heitman, J. The biology of the Cryptococcus neoformans species complex. Annu. Rev. Microbiol. 2006, 60, 69–105.
- May, R.C.; Stone, N.R.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117.
- Okurut, S.; Boulware, D.R.; Olobo, J.; Meya, D.B. Landmark clinical observations and immunopathogenesis pathways linked to HIV and Cryptococcus fatal central nervous system co-infection. Mycoses 2020, 63, 840–853.
- Shroufi, A.; Chiller, T.; Jordan, A.; Denning, D.W.; Harrison, T.S.; Govender, N.P.; Loyse, A.; Baptiste, S.; Rajasingham, R.; Boulware, D.R. Ending deaths from HIV-related cryptococcal meningitis by 2030. Lancet Infect. Dis. 2021, 21, 16–18.
- Murphy, L.S.; Lacy, A.J.; Smith, A.T.; Shah, K.S. Cryptococcal meningitis in an immunocompetent patient. Am. J. Emerg. Med. 2020, 38, e1–e2492.
- Fisher, K.M.; Montrief, T.; Ramzy, M. Cryptococcal meningitis: A review for emergency clinicians. Intern. Emerg. Med. 2021, 16, 1031–1042.
- Zhao, Y.; Ye, L.; Zhao, F.; Zhang, L.; Lu, Z.; Chu, T.; Wang, S.; Liu, Z.; Sun, Y.; Chen, M.; et al. Cryptococcus neoformans, a global threat to human health. Infect. Dis. Poverty 2023, 12, 20.
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881.
- Mourad, A.; Perfect, J.R. The war on cryptococcosis: A Review of the antifungal arsenal. Mem. Inst. Oswaldo Cruz 2018, 113, e170391.
- Kronstad, J.W.; Attarian, R.; Cadieux, B.; Choi, J.; D’souza, C.A.; Griffiths, E.J.; Geddes, J.M.; Hu, G.; Jung, W.H.; Kretschmer, M. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011, 9, 193–203.
- Botts, M.R.; Hull, C.M. Dueling in the lung: How Cryptococcus spores race the host for survival. Curr. Opin. Microbiol. 2010, 13, 437–442.
- Lenz, D.; Held, J.; Goerke, S.; Wagner, D.; Tintelnot, K.; Henneke, P.; Hufnagel, M. Primary cutaneous cryptococcosis in an eight-year-old immunocompetent child: How to treat? Klin. Padiatr. 2015, 227, 41–44.
- Dang, E.V.; Lei, S.; Radkov, A.; Volk, R.F. Secreted fungal virulence effector triggers allergic inflammation via TLR4. Nature 2022, 608, 161–167.
- Ngan, N.T.T.; Flower, B.; Day, J.N. Treatment of Cryptococcal Meningitis: How Have We Got Here and Where are We Going? Drugs 2022, 82, 1237–1249.
- Poplin, V.; Boulware, D.R.; Bahr, N.C. Methods for rapid diagnosis of meningitis etiology in adults. Biomark. Med. 2020, 14, 459–479.
- Rajasingham, R.; Wake, R.M.; Beyene, T.; Katende, A.; Letang, E.; Boulware, D.R. Cryptococcal Meningitis Diagnostics and Screening in the Era of Point-of-Care Laboratory Testing. J. Clin. Microbiol. 2019, 57, e01238-18.
- Fang, W.; Wu, J.; Cheng, M.; Zhu, X.; Du, M.; Chen, C.; Liao, W.; Zhi, K.; Pan, W. Diagnosis of invasive fungal infections: Challenges and recent developments. J. Biomed. Sci. 2023, 30, 42.
- Li, H.; Zhang, L.; Zhang, K.; Huang, Y.; Liu, Y.; Lu, X.; Liao, W.; Liu, X.; Zhang, Q.; Pan, W. Gut microbiota associated with cryptococcal meningitis and dysbiosis caused by anti-fungal treatment. Front. Microbiol. 2023, 13, 1086239.
- Yang, C.; Huang, Y.; Zhou, Y.; Zang, X.; Deng, H.; Liu, Y.; Shen, D.; Xue, X. Cryptococcus escapes host immunity: What do we know? Front. Cell. Infect. Microbiol. 2022, 12, 1041036.
- Gibson, J.F.; Johnston, S.A. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal Genet. Biol. 2015, 78, 76–86.
- Smith, D.F.; Casadevall, A. The role of melanin in fungal pathogenesis for animal hosts. Fungal Physiol. Immunopathogenesis 2019, 422, 1–30.
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The fungal cell wall: Candida, Cryptococcus, and Aspergillus species. Front. Microbiol. 2020, 10, 2993.
- Iyer, K.R.; Revie, N.M.; Fu, C.; Robbins, N.; Cowen, L.E. Treatment strategies for cryptococcal infection: Challenges, advances and future outlook. Nat. Rev. Microbiol. 2021, 19, 454–466.
- Wang, Y.; Casadevall, A. Susceptibility of melanized and nonmelanized Cryptococcus neoformans to the melanin-binding compounds trifluoperazine and chloroquine. Antimicrob. Agents Chemother. 1996, 40, 541–545.
- Baker, R.P.; Chrissian, C.; Stark, R.E.; Casadevall, A. Cryptococcus neoformans melanization incorporates multiple catecholamines to produce polytypic melanin. J. Biol. Chem. 2022, 298, 101519.
- Lee, D.; Jang, E.-H.; Lee, M.; Kim, S.-W.; Lee, Y.; Lee, K.-T.; Bahn, Y.-S. Unraveling melanin biosynthesis and signaling networks in Cryptococcus neoformans. mBio 2019, 10, e02267-19.
- Williamson, P.R.; Wakamatsu, K.; Ito, S. Melanin biosynthesis in Cryptococcus neoformans. J. Bacteriol. 1998, 180, 1570–1572.
- Li, Z.; Bi, J.; Yang, J.; Pan, J.; Sun, Z.; Zhu, X. Requirement of a Tsp2-type tetraspanin for laccase repression and stress resistance in the basidiomycete Cryptococcus neoformans. Appl. Environ. Microbiol. 2012, 78, 21–27.
- Nurudeen, T.A.; Ahearn, D.G. Regulation of melanin production by Cryptococcus neoformans. J. Clin. Microbiol. 1979, 10, 724–729.
- Erny, D.; Dokalis, N.; Mezö, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2276.e7.
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247.
- Mizgier, M.; Jarzabek-Bielecka, G. The role of diet and probiotics in prevention and treatment of bacterial vaginosis and vulvovaginal candidiasis in adolescent girls and non-pregnant women. Ginekol. Pol. 2020, 91, 412–416.
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060.
- McAleer, J.P.; Nguyen, N.L.; Chen, K.; Kumar, P. Pulmonary Th17 Antifungal Immunity Is Regulated by the Gut Microbiome. J. Immunol. 2016, 197, 97–107.
- Camacho, E.; Vij, R. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489.
- Xue, P.; Hu, G.; Jung, W.H.; Kronstad, J.W. Metals and the cell surface of Cryptococcus neoformans. Curr. Opin. Microbiol. 2023, 74, 102331.
- Chrissian, C.; Camacho, E.; Fu, M.S.; Prados-Rosales, R.; Chatterjee, S.; Cordero, R.J.B.; Lodge, J.K.; Casadevall, A.; Stark, R.E. Melanin deposition in two Cryptococcus species depends on cell-wall composition and flexibility. J. Biol. Chem. 2020, 295, 1815–1828.
- Casadevall, A.; Rosas, A.L.; Nosanchuk, J.D. Melanin and virulence in Cryptococcus neoformans. Curr. Opin. Microbiol. 2000, 3, 354–358.
- Baker, R.P.; Casadevall, A. Reciprocal modulation of ammonia and melanin production has implications for cryptococcal virulence. Nat. Commun. 2023, 14, 849.
- Nosanchuk, J.D.; Rosas, A.L.; Lee, S.C.; Casadevall, A. Melanisation of Cryptococcus neoformans in human brain tissue. Lancet 2000, 355, 2049–2050.
- Rosas, A.L.; Nosanchuk, J.D.; Feldmesser, M.; Cox, G.M.; McDade, H.C.; Casadevall, A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000, 68, 2845–2853.
- Herregodts, P.; Michotte, Y.; Ebinger, G. Regional differences in the distribution of norepinephrine and epinephrine in human cerebral cortex: A neurochemical study using HPLC and electrochemical detection. Neurosci. Lett. 1989, 98, 321–326.
- Herregodts, P.; Ebinger, G.; Michotte, Y. Distribution of monoamines in human brain: Evidence for neurochemical heterogeneity in subcortical as well as in cortical areas. Brain Res. 1991, 542, 300–306.
- Kwon-Chung, K.; Tom, W.; Costa, J. Utilization of indole compounds by Cryptococcus neoformans to produce a melanin-like pigment. J. Clin. Microbiol. 1983, 18, 1419–1421.
- Panepinto, J.C.; Williamson, P.R. Intersection of fungal fitness and virulence in Cryptococcus neoformans. FEMS Yeast Res. 2006, 6, 489–498.
- Polacheck, I.; Hearing, V.J.; Kwon-Chung, K.J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J. Bacteriol. 1982, 150, 1212–1220.
- Agirman, G.; Yu, K.B. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092.
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453.
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693 Pt B, 128–133.
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Dohnalová, L.; Lundgren, P.; Carty, J.R.; Goldstein, N.; Wenski, S.L.; Nanudorn, P.; Thiengmag, S.; Huang, K.-P.; Litichevskiy, L.; Descamps, H.C. A microbiome-dependent gut–brain pathway regulates motivation for exercise. Nature 2022, 612, 739–747.
- Diaz Heijtz, R.; Wang, S.; Anuar, F.; Qian, Y.; Björkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052.
- Fan, X.; Deng, H.; Qiu, J.; Ji, H.; Shen, X. Antibiotics-induced depression in mice via the microbiota-gut-brain axis. J. Affect. Disord. 2022, 318, 152–158.
- Grasset, E.; Burcelin, R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438.
- Fang, F.C.; Frawley, E.R.; Tapscott, T.; Vázquez-Torres, A. Bacterial Stress Responses during Host Infection. Cell Host Microbe 2016, 20, 133–143.
- Brown, A.J.; Haynes, K.; Quinn, J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr. Opin. Microbiol. 2009, 12, 384–391.
- Rutherford, J.C.; Bahn, Y.S.; van den Berg, B.; Heitman, J.; Xue, C. Nutrient and Stress Sensing in Pathogenic Yeasts. Front. Microbiol. 2019, 10, 442.




