Cryptococcus neoformans is a major cause of fungal meningitis in immunocompromised individuals. Similar to other melanized microorganisms associated with human diseases, the cell wall新型隐球菌是一种导致免疫功能低下个体患真菌性脑膜炎的主要病因。与其他与人类疾病有关的黑色素微生物一样,新型隐球菌的细胞壁相关的黑色素是其逃避宿主免疫反应的主要毒力因素。这些黑色素的水平和形成可能会受微生物群-associated melanin of C. neoformans is a major virulence factor enabling it to evade host immune responses. The levels and formation of these melanins may be influenced by the microbiota肠道-gut-brain axis. Studies have also found that C. neoformans infection can lead to dysbiosis of the human gut microbiota.脑轴的影响。近期研究还发现,新型隐球菌感染可能导致人类肠道微生物组的生态失衡。
- cryptococcal meningitis
- melanin
- catecholamines
- nutritional signals
- microbiota–gut–brain axis
1. Introductions
1. 简介
Cryptococcus neoformans is a human pathogenic fungus belonging to the phylum Basidiomycota [1][2]. It has a well-defined bipolar mating system, consisting of two different mating types, MATa and MATα [3][4], but is also capable of self-fertilization within cells of the same mating type [5][6]. C. neoformans is widely distributed in the environment, particularly in bird droppings, soil, and trees [7]. It poses a particular danger to immunocompromised hosts, such as HIV/AIDS patients and organ transplant recipients, as it can easily cause meningitis [8][9][10][11][12][13]. For instance, C. neoformans is estimated to cause approximately 223,100 new cases and 181,100 deaths annually among HIV/AIDS patients [14][15]. According to the classification by the World Health Organization (WHO), these species are classified as one of the four priority fungal pathogens affecting humans. Cryptococcus neoformans infection typically occurs through inhalation of fungal spores or desiccated yeast cells in the environment [16][17], but can also occur through skin wounds [18]. These species are able to overcome host defenses and enter the bloodstream, subsequently invading the central nervous system and causing meningitis [16][19]. The diagnosis of cryptococcal meningitis typically involves a comprehensive evaluation of clinical symptoms and signs, combined with various diagnostic methods [20][21][22][23]. These methods include assessing the patient's medical history and performing physical examinations to identify symptoms associated with cryptococcal meningitis. Lumbar puncture is a routine procedure used to obtain and analyze cerebrospinal fluid (CSF) to determine the presence of Cryptococcus species. Laboratory tests include India ink staining of the CSF, culture of Cryptococcus species, or antigen testing to confirm the presence of Cryptococcus infection. Neuroimaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) can be used to examine the brain for signs of meningitis or other abnormalities. The combined application of these methods is crucial for accurate diagnosis and exclusion of other potential causes. It has been suggested that the composition of the gut microbiota may play a potential role in the diagnosis of cryptococcal meningitis. Studies have shown that alterations in the gut microbiota may be associated with fungal infections, including Cryptococcus species [24]. C. neoformans is able to impair the accurate recognition of fungal antigens by the host and evade host immune responses, including the coordinated action of macrophages, dendritic cells, T lymphocytes, B lymphocytes, innate lymphoid cells, and cytokines [25][26]. Furthermore, C. neoformans utilizes melanin production to evade host immunity and enhance its infectivity in the host. Melanin in the cell wall of C. neoformans is a major virulence factor with multiple functions, such as resistance to oxidative stress, reduction of antifungal drug efficacy, and modulation of interactions with phagocytic cells [16][27][28]. Researchers believe that melanin may be a potential target for the development of drugs against Cryptococcus infections [29][30]. A notable feature of C. neoformans is its inability to produce melanin pigments from endogenously synthesized compounds, such as tyrosine, but instead utilizes catecholamines, such as dopamine, norepinephrine, and epinephrine, to oxidize and generate melanin in the brain tissue through the action of laccases (Lac1 and Lac2) [31][32][33]. The neurotropism of C. neoformans is closely related to the presence of catecholamines in the brain and low glucose concentrations in the central nervous system, as these conditions favor laccase production [32][34][35].
2. Cryptococcus neoformans Infection and Dysbiosis of the Gut Microbiota
The latest advances in our understanding and analysis of the gut microbiota have revealed significant impacts of alterations on human health. The gut microbiota is a complex and diverse microbial ecosystem that plays crucial roles in various aspects of host physiology. It actively participates in host immune responses, influences metabolism, promotes biosynthesis, and defends against pathogenic yeast infections [36][37][38][39]. In this regard, it has been found that the composition of the gut bacterial microbiota can influence the production of pulmonary IL-17 responses during opportunistic human fungal pathogen Aspergillus fumigatus infection in mice. Mice with specific gut bacterial compositions exhibited stronger IL-17 responses, which are critical for combating fungal infections [40]. Recently, Li and colleagues reported that C. neoformans infection induces changes in the human gut microbiota [24]. The authors performed alpha and beta diversity analyses, comparing the gut microbiota of cryptococcal meningitis patients with healthy controls. The results showed a significant reduction in alpha diversity in cryptococcal meningitis patients compared to the healthy control group, indicating gut dysbiosis. They identified a total of 72 differentially abundant bacterial species and 8 differentially abundant fungal species between these two groups (Figure
2. 新型隐球菌感染可能导致肠道微生物组的紊乱
我们对肠道微生物群的理解和分析的最新进展揭示了改变对人类健康的重大影响。肠道微生物群是一个复杂多样的微生物生态系统,在宿主生理的各个方面起着至关重要的作用。它积极参与宿主的免疫应答,影响新陈代谢,促进生物合成,并防御致病酵母菌感染[48,49,50,51]。在这种情况下,已经发现肠道细菌微生物群的组成会影响小鼠机会性人真菌病原体烟曲霉感染期间肺部IL-17反应的产生。具有特定肠道细菌组成的小鼠表现出更强大的IL-17反应,这对于对抗真菌感染至关重要[52]。 最近,Li及其同事报告说,新型隐球菌感染诱导了人类肠道微生物群的改变[34]。作者进行了α和β多样性分析,将隐球菌性脑膜炎患者的肠道微生物群与健康对照进行比较。结果显示,与健康对照组相比,隐球菌性脑膜炎患者的α多样性显着降低,表明肠道生态失调。他们总共在这两组之间鉴定了72种不同丰度的细菌和8种不同丰度的真菌物种(见图1)。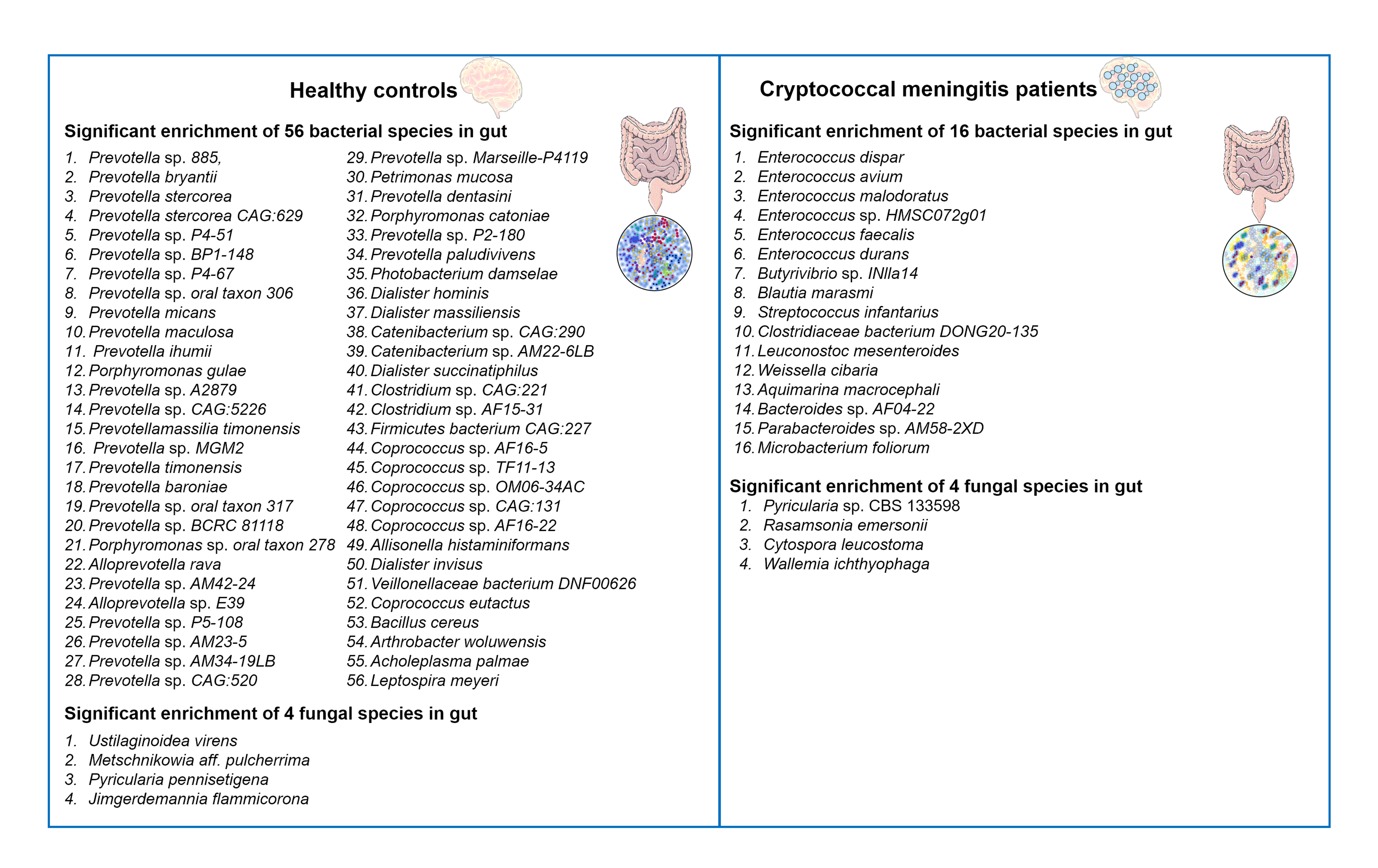
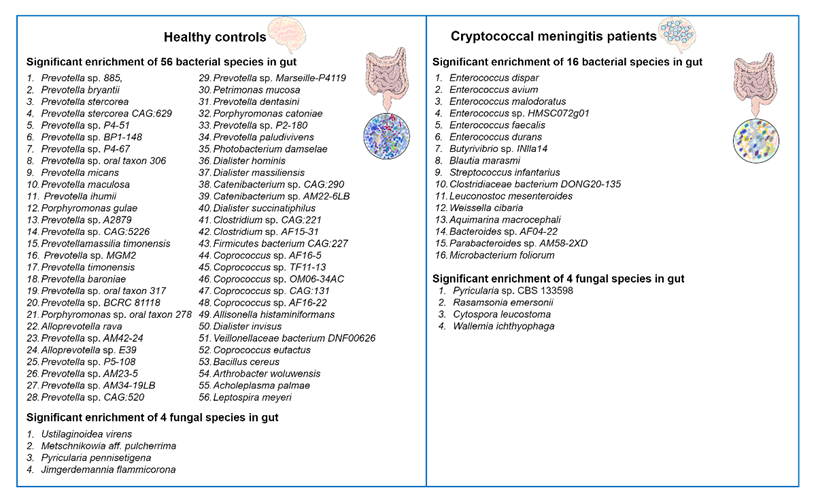 图1
图1).
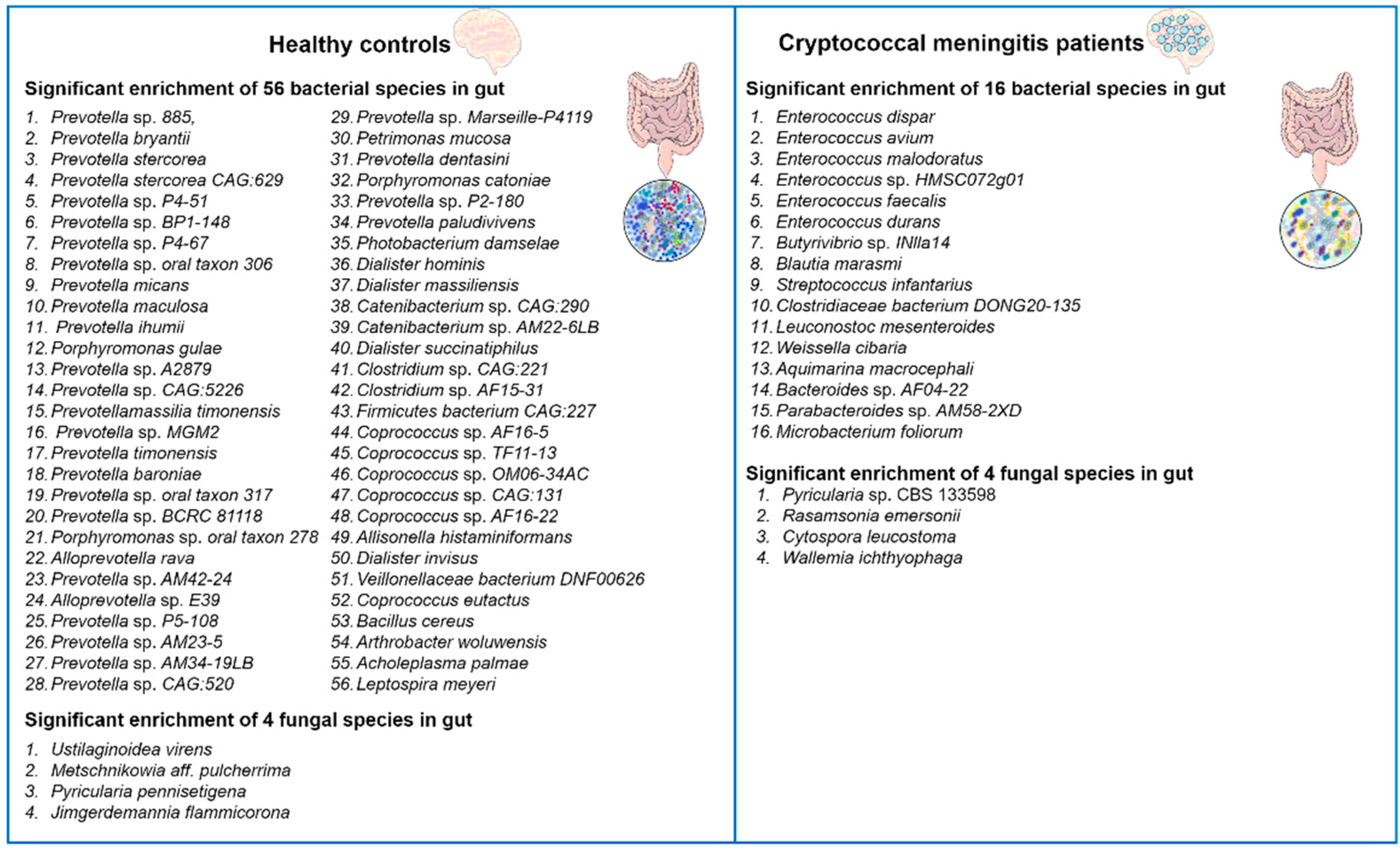
Figure 1. Changes in the diversity of gut bacterial and fungal microbiota in patients with cryptococcal meningitis. There are notable differences in the composition of gut bacterial and fungal microbiota between patients with cryptococcal meningitis and healthy controls. A total of 72 bacterial species and 8 fungal species were found to have differential abundance between these two groups [24].
2. Melanization phenomenon of Cryptococcus neoformans in human brain tissue
The deposition of melanin on the surface of Cryptococcus neoformans cells is one of its important features, and this deposition contributes to its pathogenicity [41][42]. The deposition of melanin depends on the composition and flexibility of the cell wall of C. neoformans [43]. The deposition of melanin within the cell wall provides several advantages for the pathogen [44]. Firstly, melanin acts as a protective barrier and can resist host immune reactions, including phagocytosis by immune cells. Studies have shown that melanin can inhibit the production of reactive oxygen species and reduce the activity of antifungal drugs, thereby enhancing C. neoforman resistance to host defenses. In addition, melanin is associated with the dissemination of C. neoformans within the host. Melanized fungal cells can be detected in various organs, including the brain and lungs, indicating the important role of melanization in the invasion and establishment process in different infected tissues [45]. The main sign of melanization during C. neoformans infection is the presence of acid-resistant melanin ghost particles. These particles can be isolated from infected animal and human tissues, as well as from cells cultured on agar plates [46][47]. The mammalian nervous system is a rich source of catecholamines, which are a class of nitrogen-containing diphenolic compounds, including dopamine, adrenaline, and noradrenaline, among others [48][49]. C. neoformans synthesizes melanin by using the oxidation process of exogenous catecholamine substrates catalyzed by laccase [50][51][52]. Melanized Cryptococcus neoformans cells can be detected in brain tissue samples from patients with cryptococcal meningitis [60]. The melanin synthesized by Cryptococcus neoformans in brain tissue may vary in different anatomical regions, as it can bind to multiple catecholamines simultaneously (see Figure :
3. 新型隐球菌在人脑组织中的黑化现象
新型隐球菌细胞表面的黑色素沉积是其重要的特征之一,这种沉积有利于其致病能力[55,56]。黑色素的沉积取决于新型隐球菌细胞壁的组成和柔韧性[57]。细胞壁内的黑色素沉积为病原体提供了几个优势[58]。首先,黑色素作为保护屏障,可以抵御宿主免疫反应,包括免疫细胞的吞噬作用。研究表明,黑色素可以抑制活性氧的产生,并降低抗真菌药物的活性,从而增强新型隐球菌对宿主防御的抵抗力。此外,黑色素与新型隐球菌在宿主内的传播有关。在包括大脑和肺在内的多种器官中都可以检测到黑色化的真菌细胞,这表明黑色化在感染不同组织中的侵袭和建立过程中起重要作用[59]。新型隐球菌感染期间黑色化的主要迹象是耐酸黑色素幽灵颗粒的存在。这些颗粒可以从受感染的动物和人体组织中分离出来,也可以从培养在琼脂平板上的细胞中提取[60,61]。哺乳动物神经系统是儿茶酚胺的丰富来源,而儿茶酚胺是一类含氮的二酚化合物,包括多巴胺、肾上腺素和去甲肾上腺素等神经递质[62,63]。新型隐球菌通过使用漆酶催化外源性儿茶酚胺底物的氧化过程来合成黑色素[64,65,66]。在患有隐球菌性脑膜炎的患者的脑组织样本中可以检测到黑色化的新型隐球菌细胞[60]。新型隐球菌在脑组织中合成的黑色素可能在不同解剖区域具有差异,因为它能够与多种儿茶酚胺同时结合(见图2)。这是因为不同大脑区域中这些神经递质的相对比例可能存在较大差异[62,63]。化学结构对添加到培养基中的基质决定了合成黑色素类型的可变性。值得注意的是,Baker等人对新型隐球菌利用人脑中的儿茶酚胺混合物(0.6 mM多巴胺,0.33 mM去甲肾上腺素和0.07 mM肾上腺素)产生不同形式的黑色素的能力进行了重要观察。这种黑色素可以对抗紫外线和氧化剂的作用。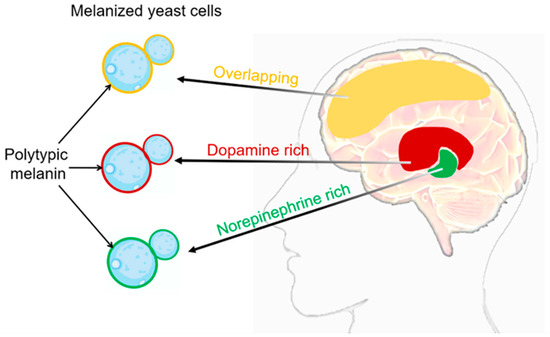
). This is because the relative proportions of these neurotransmitters may vary greatly in different brain regions [48][49]. The chemical structure determines the variability of the types of melanin synthesized when added to the culture medium. It is worth noting that Baker et al. made important observations on the ability of Cryptococcus neoformans to produce different forms of melanin using a mixture of catecholamines in the human brain (0.6 mM dopamine, 0.33 mM adrenaline, and 0.07 mM noradrenaline). This melanin can provide protection against UV radiation and oxidants.

Figure 2. Melanization of C. neoformans in different brain tissues. The composition of melanin produced during the infection process may vary depending on the composition of catecholamines in the brain tissue. It is likely that melanin synthesis in vivo is generated through the polymerization of various precursor compounds [27]. Distribution of catecholamines in the brain: the red region represents dopamine enrichment, the green region represents norepinephrine enrichment, and the yellow region represents the overlap of dopamine and norepinephrine. In the cell wall of C. neoformans, the melanin produced in different brain regions can be visualized as red, green, and yellow.
4. Potential impact of the gut microbiota on melanization of Cryptococcus neoformans
4.1. The gut microbiota may influence the levels of melanin substrates
The Gut-brain axis is a bidirectional communication system connecting the gut microbiota and the central nervous system [53][54]. It is generally believed that the gut microbiota has the ability to influence various aspects of brain function, such as emotions, behavior, and cognition [55][56]. This influence may occur through different pathways, such as affecting the production of neurotransmitters and other signaling molecules that can impact the function of the central nervous system [70, 72]. While there is no direct evidence linking the gut-brain axis to the synthesis of melanin in C. neoformans, there is evidence suggesting that the gut microbiota may influence the levels of catecholamines (such as dopamine and norepinephrine) (see Figure :
4. 肠道微生物组对新型隐球菌黑化的潜在影响
4.1. 肠道微生物组可能会影响黑色素底物的水平
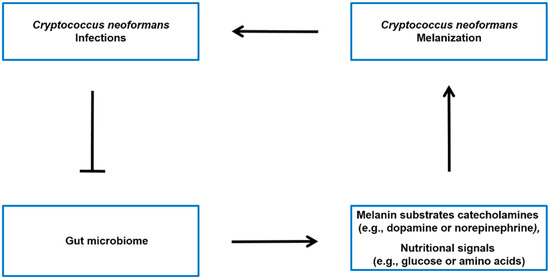
), which are precursors for melanin synthesis in C. neoformans.
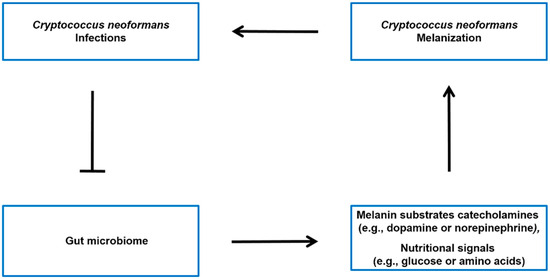
Figure: 3. Potential model of the interplay between C. neoformans infection, gut microbiota, and host molecules. It has been observed that there is an association between C. neoformans infection and alterations in the gut microbiota [24]. The microbiota-gut-brain axis may regulate the levels of melanin sunstrate catecholamines (such as dopamine or norepinephrine) [57][58][59], as well as modulate the nutritional signals (such as glucose or amino acids) that influence Cryptococcus neoformans melanin production in the brain [60].
4.2. The gut microbiota may influence the generation of Cryptococcus neoformans melanin by modulating nutritional signals
The ability to rapidly adapt to fluctuating external conditions is crucial for the survival and proliferation of microorganisms. This is especially important for pathogenic microorganisms like C. neoformans, as they need to adapt to the transition from the environment to the host and initiate appropriate responses to establish infection. The host environment presents various challenging conditions, such as changes in available nutrients, oxygen levels, pH, and temperature, as well as potential threats from the host immune response [61][62]. It is noteworthy that the mechanisms for adapting to nutrient availability not only contribute to microbial proliferation but also play a role in regulating their virulence [63].
