
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Attilio Galhardo | -- | 1311 | 2023-07-27 19:00:44 | | | |
| 2 | Dean Liu | Meta information modification | 1311 | 2023-07-28 05:28:55 | | |
Video Upload Options
Within the last two decades, transcatheter aortic valve replacement (TAVR) has transformed the treatment strategy for symptomatic severe aortic stenosis (AS), representing a less invasive alternative to traditional open-chest surgery. With time, advances in device features, imaging planning, and implantation techniques have contributed to an improvement in safety as well as a reduction in procedural complications. This has led to the expansion of TAVR to lower-risk patients, where TAVR has shown favorable outcomes compared to surgical aortic valve replacement (SAVR). As TAVR expands to younger and lower-risk patients with longer life expectancies, the need for reintervention for failing transcatheter heart valves is expected to increase. Redo-TAVR has gained increasing relevance in the lifetime management of AS as one of the treatment strategies available for structural valve dysfunction (SVD).
1. Introduction
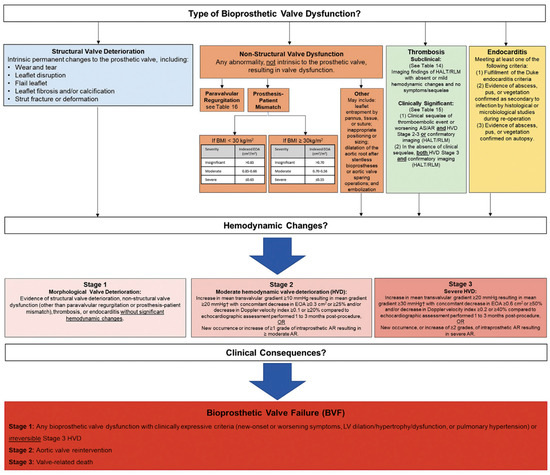
2. Bioprosthetic Valve Dysfunction and Structural Valve Deterioration Definitions
3. TAVR Explantation
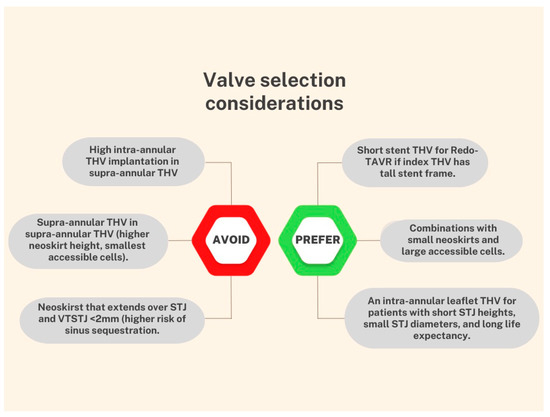
4. TAVR-in-TAVR: Procedural Complications
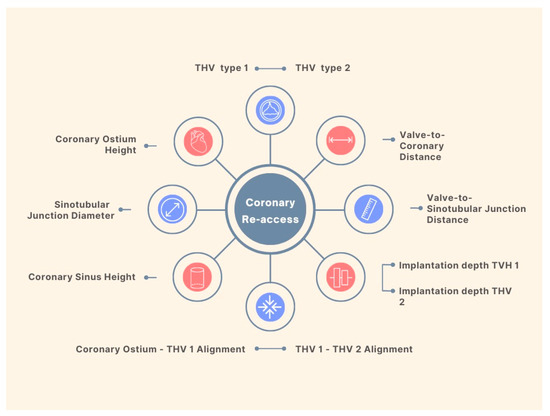
Coronary Obstruction
References
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008.
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607.
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620.
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705.
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516.
- Faroux, L.; Alperi, A.; Muntané-Carol, G.; Rodes-Cabau, J. Safety and efficacy of repeat transcatheter aortic valve replacement for the treatment of transcatheter prosthesis dysfunction. Expert Rev. Med. Devices 2020, 17, 1303–1310.
- Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; Blackstone, E.H.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. J. Am. Coll. Cardiol. 2021, 77, 2717–2746.
- Bapat, V.N.; Zaid, S.; Fukuhara, S.; Saha, S.; Vitanova, K.; Kiefer, P.; Squiers, J.J.; Voisine, P.; Pirelli, L.; von Ballmoos, M.W.; et al. Surgical Explantation After TAVR Failure: Mid-Term Outcomes From the EXPLANT-TAVR International Registry. JACC Cardiovasc. Interv. 2021, 14, 1978–1991.
- Fukuhara, S.; Nguyen, C.T.N.; Yang, B.; Patel, H.J.; Ailawadi, G.; Kim, K.M.; Deeb, G.M. Surgical Explantation of Transcatheter Aortic Bioprostheses: Balloon vs Self-Expandable Devices. Ann. Thorac. Surg. 2022, 113, 138–145.
- Fukuhara, S.; Brescia, A.A.; Deeb, G.M. Surgical Explantation of Transcatheter Aortic Bioprostheses An Analysis From the Society of Thoracic Surgeons Database. Circulation 2020, 142, 2285–2287.
- Zaid, S.; Fukuhara, S.; Marin-Cuartas, M.; Backer, O.D.; Bhadra, O.; Grubb, K.; Shih, E.; Meier, D.; Goel, S.; Tang, G. TCT-399 Impact of Mechanism of TAVR Failure on Outcomes after Reintervention for Failed TAVR: Insights From the EXPLANTORREDO-TAVR International Registry. J. Am. Coll. Cardiol. 2022, 80, B161–B162.
- Vitanova, K.; Zaid, S.; Tang, G.H.L.; Kaneko, T.; Bapat, V.N.; Modine, T.; Denti, P. Aortic valve versus root surgery after failed transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2022. online ahead of print.
- Faroux, L.; Guimaraes, L.; Wintzer-Wehekind, J.; Junquera, L.; Ferreira-Neto, A.N.; del Val, D.; Muntané-Carol, G.; Mohammadi, S.; Paradis, J.M.; Rodés-Cabau, J. Coronary Artery Disease and Transcatheter Aortic Valve Replacement: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 362–372.
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 143, e72–e227.
- Altibi, A.M.; Hammad, F.; Patel, J.; Song, H.K.; Golwala, H.; Zahr, F.E.; Rahmouni, H. Clinical Outcomes of Revascularization with Percutaneous Coronary Intervention Prior to Transcatheter Aortic Valve Replacement: A Comprehensive Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101339.
- Patterson, T.; Clayton, T.; Dodd, M.; Khawaja, Z.; Morice, M.C.; Wilson, K.; Kim, W.K.; Meneveau, N.; Hambrecht, R.; Byrne, J.; et al. ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION): A Randomized Clinical Trial. JACC Cardiovasc. Interv. 2021, 14, 1965–1974.
- Stefanini, G.G.; Cerrato, E.; Pivato, C.A.; Joner, M.; Testa, L.; Rheude, T.; Pilgrim, T.; Pavani, M.; Brouwer, J.; Lopez Otero, D.; et al. Unplanned Percutaneous Coronary Revascularization After TAVR: A Multicenter International Registry. JACC Cardiovasc. Interv. 2021, 14, 198–207.
- Kim, W.K.; Pellegrini, C.; Ludwig, S.; Möllmann, H.; Leuschner, F.; Makkar, R.; Leick, J.; Amat-Santos, I.J.; Dörr, O.; Breitbart, P.; et al. Feasibility of Coronary Access in Patients With Acute Coronary Syndrome and Previous TAVR. JACC Cardiovasc. Interv. 2021, 14, 1578–1590.
- de Backer, O.; Landes, U.; Fuchs, A.; Yoon, S.H.; Mathiassen, O.N.; Sedaghat, A.; Kim, W.K.; Pilgrim, T.; Buzzatti, N.; Ruile, P.; et al. Coronary Access after TAVR-in-TAVR as Evaluated by Multidetector Computed Tomography. JACC Cardiovasc. Interv. 2020, 13, 2528–2538.
- Meier, D.; Akodad, M.; Landes, U.; Barlow, A.M.; Chatfield, A.G.; Lai, A.; Tzimas, G.; Tang, G.H.L.; Puehler, T.; Lutter, G.; et al. Coronary Access Following Redo TAVR: Impact of THV Design, Implant Technique, and Cell Misalignment. JACC Cardiovasc. Interv. 2022, 15, 1519–1531.
- Wijesinghe, N.; Ye, J.; Rodés-Cabau, J.; Cheung, A.; Velianou, J.L.; Natarajan, M.K.; Dumont, E.; Nietlispach, F.; Gurvitch, R.; Wood, D.A.; et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc. Interv. 2010, 3, 1122–1125.
- Mesnier, J.; Panagides, V.; Nuche, J.; Rodés-Cabau, J. Evolving Indications of Transcatheter Aortic Valve Replacement—Where Are We Now, and Where Are We Going. J. Clin. Med. 2022, 11, 3090.
- Chen, F.; Xiong, T.; Li, Y.; Wang, X.; Zhu, Z.; Yao, Y.; Ou, Y.; Li, X.; Wei, X.; Zhao, Z.; et al. Risk of Coronary Obstruction During Redo-TAVR in Patients With Bicuspid Versus Tricuspid Aortic Valve Stenosis. JACC Cardiovasc. Interv. 2022, 15, 712–724.




