Within the last two decades, transcatheter aortic valve replacement (TAVR) has transformed the treatment strategy for symptomatic severe aortic stenosis (AS), representing a less invasive alternative to traditional open-chest surgery. With time, advances in device features, imaging planning, and implantation techniques have contributed to an improvement in safety as well as a reduction in procedural complications. This has led to the expansion of TAVR to lower-risk patients, where TAVR has shown favorable outcomes compared to surgical aortic valve replacement (SAVR). As TAVR expands to younger and lower-risk patients with longer life expectancies, the need for reintervention for failing transcatheter heart valves is expected to increase. Redo-TAVR has gained increasing relevance in the lifetime management of AS as one of the treatment strategies available for structural valve dysfunction (SVD).
- redo-TAVR
- ViV-TAVR
- bioprosthetic valve degeneration
- coronary obstruction
1. Introduction
2. Bioprosthetic Valve Dysfunction and Structural Valve Deterioration Definitions
The Valve Academic Research Consortium (VARC) published its most recent updates in response to the exponential growth in the number of transcatheter and surgical aortic valve interventions in order to standardize clinical endpoints for both procedures [7]. Bioprosthetic Valve Dysfunction (BVD) and Structural Valve Deterioration (SVD) were included as novel endpoints in the VARC-3 document (Figure 1). BVD was subcategorized into four groups: structural valve deterioration (the dysfunction is related to an intrinsic permanent change in the prosthetic valve, such as wear and tear, leaflet disruption, flail leaflet, leaflet fibrosis and/or calcification and strut fracture), non-structural valve dysfunction (such as paravalvular regurgitation, prosthesis-patient mismatch, leaflet entrapment by pannus, tissue or suture, inappropriate positioning, or sizing), thrombosis, and endocarditis. Three stages of BVD were proposed according to hemodynamic changes: Stage 1: morphological valve deterioration, where no hemodynamic change is present; Stage 2: moderate hemodynamic deterioration; and Stage 3: severe hemodynamic valve deterioration.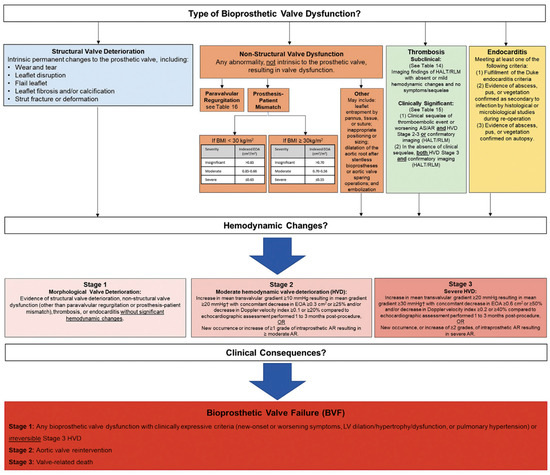
3. TAVR Explantation
Prosthesis failure mechanism, patient’s anatomical characteristics, age, comorbidities, and life expectancy are among the factors that should be considered when deciding what is the best alternative for a failed transcatheter bioprosthetic valve. TAVR explantation consists of surgically removing a transcatheter aortic valve device from the patient’s body. It requires a skilled surgical team and is associated with an increased risk of complications. Nevertheless, for selected patients, this may be the strategy of choice as a second intervention. Data from the multicenter, international EXPLANT-TAVR registry reported elevated mortality and stroke rates regardless of the procedure’s timing and primary indication for TAVR explantation [8]. According to that study, after one year, the overall mortality rate was 28.5% and the stroke rate was 18.7%. Up to 63% of patients undergoing THV explantation needed concomitant procedures, such as aortic repair, mitral or tricuspid interventions, or coronary artery bypass grafting [9][10][9,10]. In terms of device type, 30-day mortality and need for any concomitant cardiac procedures at the time of TAVR explant were comparable between self- (SEV) and balloon-expandable (BEV) valves, with a higher rate of ascending aortic repair related to self-expandable devices (22% vs. 9%; p < 0.001) [9]. Newly released results from the EXPLANTORREDO-TAVR registry, including 542 patients from 29 centers who underwent TAVR reintervention (TAVR-explant or Redo-TAVR) due to THV failure, suggested that neither the mechanism of degeneration (SVD or non-SVD) nor the device design (BEV or SEV) significantly impacted mortality after TAVR reintervention [11]. As for outcomes of TAVR explant versus aortic root replacement after failed TAVR, overall survival at a mean follow-up of 6.9 months was 81.2%, with no differences in in-hospital, 30-day, and 1-year stroke and mortality rates between the two groups [12].4. TAVR-in-TAVR: Procedural Complications
Coronary Obstruction
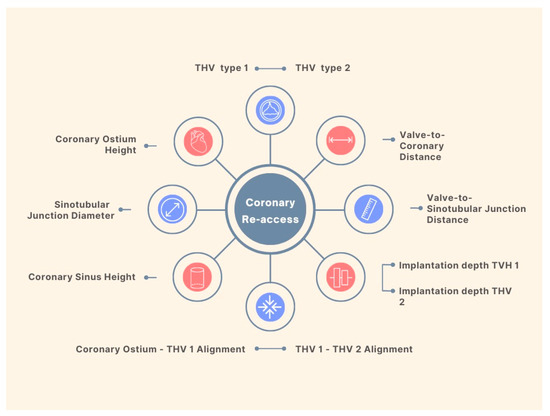
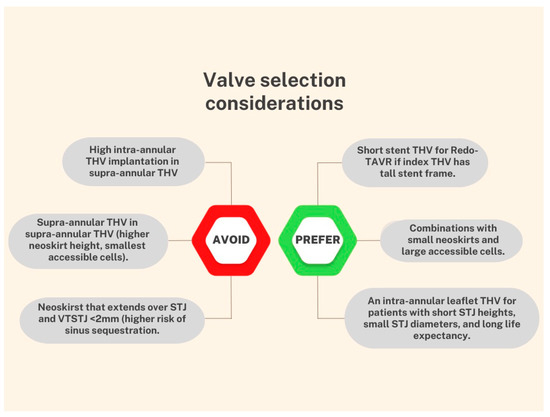
Coronary artery disease is one of the most frequent comorbidities among TAVR candidates and the optimal management in this population still lacks definitive data [13][19]. A few scenarios are supported by current guidelines for percutaneous coronary intervention (PCI) in patients undergoing TAVR; however, the level of evidence is limited, and the recommendation is only moderately strong [14][20]. Meanwhile, recent evidence showed no benefit in short- or mid-term mortality with routine PCI pre-TAVR, with a trend towards an increased risk for bleeding events [15][16][21,22]. Data from a multicenter registry indicate a rate of 0.9% of unplanned percutaneous coronary revascularization after TAVR [17][23]. This number is expected to increase given the implantation of THV in patients with lower surgical risk, and a relatively younger population. Although registries report high success rates of coronary cannulation in patients with acute coronary syndromes and THVs [18][24], the feasibility of coronary access after a Redo-TAVR remains a big concern. Factors impacting coronary re-access after Redo-TAVR procedures are summarized in Figure 2.