
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Olga V. Razumova | -- | 3753 | 2023-03-14 11:59:15 | | | |
| 2 | Lindsay Dong | -28 word(s) | 3725 | 2023-03-15 06:51:06 | | | | |
| 3 | Lindsay Dong | -3 word(s) | 3722 | 2023-03-15 06:53:16 | | |
Video Upload Options
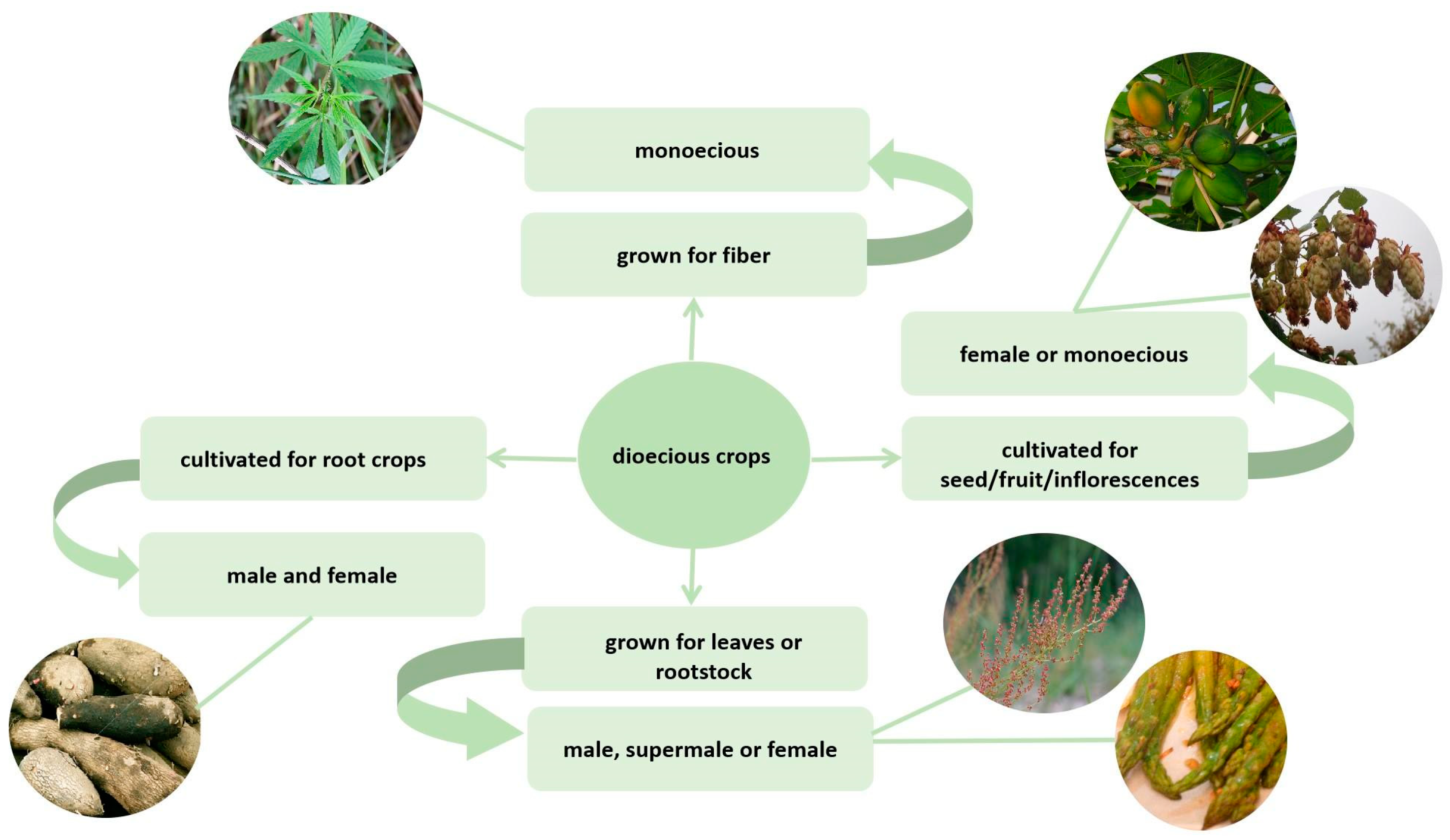
Unlike in animals, dioecy among flowering plants is a rare phenomenon. The vast majority of angiosperm species have a bisexual flower that combines male (androecium) and female (gynoecium) reproductive organs. However, about a quarter of species have dioecious flowers, which can be located within the same plant (monoecious) or on different plants (dioecious). The development of sequencing techniques, bioinformatics, and molecular biology has led to an increase in interest in the sex determination mechanisms among plants. It is noteworthy that a significant number of dioecious plants have economic value. At the same time, dioeciousness often complicates the growing process. This fact increases the relevance of studies on dioecious crops.
1. Introduction
2. Features of the Dioecious Crop Cultivation
2.1. Sex Chromosomes and Sex Determination in Herbaceous Crops
2.1.1. Asparagus
2.1.2. Sorrel
The Rumex genus is one of the model groups for studying sex determination in plants [16]. Therefore, sex determination and sex chromosomes are widely studied in a number of Rumex species. Among these species, there are various systems for determining sex and sex chromosomes (completely hermaphroditic species, monoecious species with XY chromosomes and an active Y chromosome, and species with X:A balance sex determination, including multichromosomal species with the XY1Y2 system). Species such as R. acetosa, R. acetosella, R. hastatalus, and R. suffruticosus are dioecious and widely studied. The R. acetosa karyotype consists of 12 autosomes and sex chromosomes (XX in female plants and the XY1Y2 multichromosomal system in male plants) [17][18][19][20]. The sex chromosomes are the largest in the karyotype. Two Y chromosomes contain 26% of the total DNA. Sorrel Y chromosomes are heterochromatic and rich in repetitive DNA sequences [21]. In 1999, a group of tandem repeats specific to male sex chromosomes were isolated and called RAYSI (Rumex acetosa Y-chromosome-specific I) [22]. After that, a number of satellite DNA repeats specific for sorrel Y chromosomes were described. The repeat sequences of RAYSII and RAYSIII are AT-rich and show about 60% homology with RAYSI [17]. Repeat satDNA RAE180 is localized on two Y chromosomes and is also present on a pair of autosomes. [23].
2.1.3. Spinacia
2.1.4. Hemp
2.1.5. Betel
Betel rarely blooms, but there have been reports of economically significant differences in the varieties of male and female plants [39]. There is very little information about the chromosome numbers and the level of ploidy of this species. The main number of chromosomes of this species is x = 13; however, polyploid rows are reported in this culture. Apparently, plants with a high level of ploidy are mainly grown. It was shown that female plants are tetraploid and have 52 (2n = 4x = 52) chromosomes in their karyotype, while male plants are triploid and have 39 chromosomes (2n = 3x = 39) [40]. Sex markers have been created for betel plants, mainly to accompany the breeding process, which is currently not very developed due to the complexity in the biology of the culture [39][41][42].
2.1.6. Yams
Yams (Dioscorea rotundata) are one of the most important crops in a number of countries, primarily in the African continent, where Nigeria, Ghana, Côte d’Ivoire, and Benin are considered leaders in the production of yams [43]. The plant is grown for the production of tubers, which are then used for food. Normally, D. rotundata is a dioecious species; however, cases of monoecious occurrence have been observed. Despite the huge role of this crop in African countries, the selection of this species is difficult, largely due to its dioecious nature and problems with flowering (it is predominantly a vegetatively propagated crop) [44][45]. In addition, a change in the sex of plants under the influence of the environment is repeatedly observed during the growing season, which also complicates the breeding process of this crop [46]. The identification of sex chromosomes in yams is also difficult [47]. However, using modern sequencing techniques, the ZZ/ZW sex determination system has been demonstrated [47]. In 2020, the first yam genome obtained as long reads using the Oxford Nanopore Technologies technique was presented [48].
2.2. Sex Chromosomes and Sex Determination of Perennial Tree Crops Grown for Fruits
2.2.1. Papaya
The papaya sex chromosomes were the second to be fully sequenced after the human sex chromosomes [50]. They are evolutionarily young and outwardly practically homomorphic [51][52]. One of the features of papaya is the presence of hermaphroditic plants—in fact, it is a trioecious species, not a dioecious species. There are two types of Y chromosomes in this species. The first type is represented by the Y chromosome, which is found in male plants. The second type is represented by the Yh chromosome, which causes the hermaphroditism of flowers. At the same time, the YY, YYh, and YhYh genotypes are lethal [53][54]. The non-recombining region on the Y chromosome of papaya was formed as a result of two inversions of retrotransposons and is 8.1 Mb, while the size of the analogous region of the X chromosome is 3.5 Mb. All genes in non-recombining regions have been annotated [55]. Wild papaya is generally dioecious but cultivated as a rule of the gynodioecy cultivar [56]. It has been shown that evolutionarily hermaphroditic plants have been fixed for about 4000 years during domestication [57]. In addition to differences in the ease of cultivation (when growing hermaphroditic plants, male pollinating plants that do not produce fruits are not needed) between female and hermaphroditic plants, there is dimorphism in the shape of the fruits. Fruits from hermaphrodite flowers are more elongated and commercial producers prefer them, rejecting the more rounded fruits of female plants [50]. However, plants can change the sex of their flowers during the growing process. Hermaphroditic flowers can stop the development of carpels, turning into functionally male flowers under the influence of environmental factors such as drought or high temperatures [58][59][60].
2.2.2. Vitis
It has been shown that the emergence of hermaphroditic forms occurred about 6000–8000 years ago at the time of the domestication of wild grapes [5][61][62]. Like papaya, grapes have three sex-determining loci—Y (M) is male, X (F) is female, and Yh (H) is hermaphroditic. Unlike papaya, the HH, HM, or MM genotypes are viable in grapes. Three sex-linked grape genes (ViviPLATZ, VviFSEX, and APRT3) have been described, and the expression of each differs in hermaphroditic plants [62].
2.2.3. Diospyros
It is an autopolyploid species with 2n = 90 or 135 chromosomes (2n = 6x; 2n = 9x; x = 15) [63]. Despite the presence of at least one Y chromosome in the karyotype, this species is usually monoecious or has completely female plants, with rare male flowers [64]. Recent studies have shown that in the dioecious diploid species D. lotius L. (a close relative of D. kaki), the OGI gene located on the Y chromosome is responsible for sex formation. It encodes a small RNA that targets the autosomal MeGl gene, regulating anther fertility in a dose-dependent manner. In polyploid species (D. kaki, in particular), there is an insertion of a retroelement, named Kali, in the promoter region of the OGI gene that prevents the synthesis of small RNA and promotes the development of monoecy.
2.2.4. Pistachio
2.2.5. Hippophae
he sea buckthorn karyotype consists of 24 chromosomes (22 autosomes and a pair of X/Y sex chromosomes) [69]. In the genome (about 2.6 pg in size), researchers have observed a uniquely large number of satellites that may turn out to be good cytogenetic markers [69][70][71]. The development of molecular markers to determine the sex of sea buckthorn in the early stages of cultivation has been quite successful, but the experiments have shown the unstable operation of these markers, which may be due to sea buckthorn’s polymorphism [72][73][74]. Morphologically, male and female plants can only be distinguished by their generative buds after flowering age has been reached. At the same time, mainly female plants are needed in agricultural production. Additionally, breeding is carried out independently for male pollinating plants and female plants [75]. These facts demonstrate the need for a deeper study of the genetic determination of the sex in sea buckthorn.
2.2.6. Humulus
Humulus lupulus L. is a dioecious woody vine of the Cannabaceae family that is of great importance in brewing. Hops are grown for the female buds, which contain substances that create the characteristic taste, aroma and bitterness of beer. Due to the popularity of this drink, hops are cultivated on every continent except Antarctica. The hop karyotype consists of 18 autosomes and a pair of sex chromosomes (XX/XY). The Y chromosome is the smallest in the karyotype, which may indicate its degeneration and evolutionary antiquity [76][77][78]. In commercial cultivation, the female varieties are vegetatively propagated. A very important task is to prevent the pollination of inflorescences, since setting seeds spoils the taste of beer [79]. Hop is a wind-pollinated crop with light pollen, so just one male plant in the area can cause a lot of economic damage [80]. To prevent pollination, all male plants in a nursery and nearby wild populations must be eradicated. As a part of this procedure, molecular genetic markers are used to identify sex in the early stages of ontogenesis [81][82][83]. In hop production, seedless triploid varieties are also cultivated. They are more productive and have a high growth rate [84][85].
2.2.7. Date Palm
2.2.8. Myristica
2.2.9. Actinidia
Morphologically, the flowers look bisexual, but female plants form flowers with sterile pollen while male plants have an underdeveloped and non-functioning pistil [97]. However, some species sometimes have bisexual flowers. Hermaphroditic flowers have been observed in A. arguta, A. chinensis, A. deliciosa, and A. eriantha Benth. [98]. Kiwifruit has only been cultivated for just over 100 years, but it has achieved great popularity around the world, and its production is about 4 million tons per year [43]. Polyploidy is widespread in species of the Actinidia genus [99]. Chromosomal numbers range from 2n = 58 to 2n = 174 (x = 29) [100]. Polyploidy does not appear to affect sex in kiwifruit. Male plants have at least one Y chromosome in the karyotype, and female plants have XX [101][102]. The sex chromosomes are small and homomorphic, with a small SDR [103][104]. The mechanism of sex determination has been widely studied. This mechanism is based on two genes (the Shy Girl gene (SyGI), the dominant suppressor of carpel development, and the Friendly Boy gene (FrBy), expressed in tapetum cells) [105]. Currently, the kiwifruit genome is actively being studied, and genetic maps and molecular markers are being created to aid the breeding of this crop [106][107][108][109]. However, hermaphroditism can be determined by additional genetic factors, which creates difficulties for sex determination based on molecular markers [109].
2.2.10. Ilex
2.2.11. Chinese Bayberry
Chinese bayberry (Morella rubra Lour.) is widely cultivated in China for fruits. It is the only edible species of the Myricaceae family [118]. The chromosome number is 2n = 16, and the heterogametic sex is presumably female (ZW chromosome system) [119]. A comparison of the sequenced genomes of male and female plants revealed a small region (59 kb) on chromosome 8 that is specific to female plants [120]. The differential expression of some genes, presumably associated with the development of sex in this species, was also shown [121].
3. Conclusions
References
- Renner, S.S. The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 2014, 101, 1588–1596.
- Bachtrog, D.; Mank, J.E.; Peichel, C.L.; Kirkpatrick, M.; Otto, S.P.; Ashman, T.-L.; Hahn, M.W.; Kitano, J.; Mayrose, I.; Ming, R.; et al. Sex Determination: Why So Many Ways of Doing It? PLoS Biol. 2014, 12, e1001899.
- Vyskot, B.; Hobza, R. The genomics of plant sex chromosomes. Plant Sci. 2015, 236, 126–135.
- Montalvão, A.P.L.; Kersten, B.; Fladung, M.; Müller, N.A. The Diversity and Dynamics of Sex Determination in Dioecious Plants. Front. Plant Sci. 2021, 11, 580488.
- Picq, S.; Santoni, S.; Lacombe, T.; Latreille, M.; Weber, A.; Ardisson, M.; Ivorra, S.; Maghradze, D.; Arroyo-Garcia, R.; Chatelet, P.; et al. A small XY chromosomal region explains sex determination in wild dioecious V. vinifera and the reversal to hermaphroditism in domesticated grapevines. BMC Plant Biol. 2014, 14, 229.
- Schaefer, H.; Renner, S.S. A three-genome phylogeny of Momordica (Cucurbitaceae) suggests seven returns from dioecy to monoecy and recent long-distance dispersal to Asia. Mol. Phylogenetics Evol. 2010, 54, 553–560.
- Weiblen, G.D. Phylogenetic relationships of functionally dioecious FICUS (Moraceae) based on ribosomal DNA sequences and morphology. Am. J. Bot. 2000, 87, 1342–1357.
- Testolin, R.; Pilkington, S.M.; Akagi, T. Editorial: Dioecy in Fruit Crops: The Gender Rise and Decline and Its Agronomic Impact. Front. Plant Sci. 2021, 12, 719588.
- Flory, W.S. Genetic and cytological investigations on Asparagus officinalis L. Genetics 1932, 17, 432–467.
- Deng, C.-L.; Qin, R.-Y.; Wang, N.-N.; Cao, Y.; Gao, J.; Gao, W.-J.; Lu, L.-D. Karyotype of asparagus by physical mapping of 45S and 5S rDNA by FISH. J. Genet. 2012, 91, 209–212.
- Löptien, H. Giemsa-Banden auf Mitosechromosomen des Spargels (Asparagus officinalis L.) und des Spinats (Spinacia oleracea L.). Z Pflanz. 1976, 76, 225–230.
- Ming, R.; Bendahmane, A.; Renner, S.S. Sex Chromosomes in Land Plants. Annu. Rev. Plant Biol. 2011, 62, 485–514.
- Harkess, A.; Zhou, J.; Xu, C.; Bowers, J.E.; Van der Hulst, R.; Ayyampalayam, S.; Mercati, F.; Riccardi, P.; McKain, M.R.; Kakrana, A.; et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 2017, 8, 1–10.
- Harkess, A.; Mercati, F.; Shan, H.; Sunseri, F.; Falavigna, A.; Leebens-Mack, J. Sex-biased gene expression in dioecious garden asparagus (Asparagus officinalis). New Phytol. 2015, 207, 883–892.
- Li, S.-F.; Lv, C.-C.; Lan, L.-N.; Jiang, K.-L.; Zhang, Y.-L.; Li, N.; Deng, C.-L.; Gao, W.-J. DNA methylation is involved in sexual differentiation and sex chromosome evolution in the dioecious plant garden asparagus. Hortic. Res. 2021, 8, 198.
- Cuñado, N.; Navajas-Pérez, R.; de la Herrán, R.; Rejón, C.R.; Rejón, M.R.; Santos, J.L.; Garrido-Ramos, M.A. The evolution of sex chromosomes in the genus Rumex (Polygonaceae): Identification of a new species with heteromorphic sex chromosomes. Chromosom. Res. 2007, 15, 825–833.
- Mariotti, B.; Manzano, S.; Kejnovský, E.; Vyskot, B.; Jamilena, M. Accumulation of Y-specific satellite DNAs during the evolution of Rumex acetosa sex chromosomes. Mol. Genet. Genom. 2008, 281, 249–259.
- Rejón, C.R.; Jamilena, M.; Ramos, M.G.; Parker, J.S. Cytogenetic and molecular analysis of the multiple sex chromosome system of Rumex acetosa. Heredity 1994, 72, 209–215.
- Takenaka, Y. On the Special Autosomes with Reference to the Sex-determination of Rumex acetosa L. Cytologia 1937, 2, 995–1002.
- Kihara, H.; Ono, T. The sex-chromosomes of rumex acetosa. Mol. Genet. Genom. 1925, 39, 1–7.
- Wilby, A.S.; Parker, J.S. Recurrent patterns of chromosome variation in a species group. Heredity 1988, 61, 55–62.
- Shibata, F.; Hizume, M.; Kuroki, Y. Chromosome painting of Y chromosomes and isolation of a Y chromosome-specific repetitive sequence in the dioecious plant Rumex acetosa. Chromosoma 1999, 108, 266–270.
- Shibata, F.; Hizume, M.; Kuroki, Y. Molecular cytogenetic analysis of supernumerary heterochromatic segments in Rumex acetosa. Genome 2000, 43, 391–397.
- She, H.; Xu, Z.; Zhang, H.; Li, G.; Wu, J.; Wang, X.; Li, Y.; Liu, Z.; Qian, W. Identification of a male-specific region (MSR) in Spinacia oleracea. Hortic. Plant J. 2021, 7, 341–346.
- She, H.; Liu, Z.; Xu, Z.; Zhang, H.; Cheng, F.; Wang, X.; Qian, W. The female (XX) and male (YY) genomes provide insights into the sex determination mechanism in spinach. bioRxiv 2020.
- Vitale, J.J.; Freeman, D.C. Secondary sex characteristics in Spinacia oleracea L.: Quantitative evidence for the existence of at least three sexual morphs. Am. J. Bot. 1985, 72, 1061–1066.
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358.
- Faux, A.-M.; Draye, X.; Lambert, R.; D’Andrimont, R.; Raulier, P.; Bertin, P. The relationship of stem and seed yields to flowering phenology and sex expression in monoecious hemp (Cannabis sativa L.). Eur. J. Agron. 2013, 47, 11–22.
- Baldini, M.; Ferfuia, C.; Piani, B.; Sepulcri, A.; Dorigo, G.; Zuliani, F.; Danuso, F.; Cattivello, C. The Performance and Potentiality of Monoecious Hemp (Cannabis sativa L.) Cultivars as a Multipurpose Crop. Agronomy 2018, 8, 162.
- Razumova, O.V.; Alexandrov, O.S.; Divashuk, M.G.; Sukhorada, T.I.; Karlov, G.I. Molecular cytogenetic analysis of monoecious hemp (Cannabis sativa L.) cultivars reveals its karyotype variations and sex chromosomes constitution. Protoplasma 2015, 253, 895–901.
- Faux, A.-M.; Berhin, A.; Dauguet, N.; Bertin, P. Sex chromosomes and quantitative sex expression in monoecious hemp (Cannabis sativa L.). Euphytica 2013, 196, 183–197.
- Mandolino, G.; Carboni, A.; Forapani, S.; Faeti, V.; Ranalli, P. Identification of DNA markers linked to the male sex in dioecious hemp (Cannabis sativa L.). Theor. Appl. Genet. 1999, 98, 86–92.
- Törjék, O.; Bucherna, N.; Kiss, E.; Homoki, H.; Finta-Korpelová, Z.; Bócsa, I.; Nagy, I.; Heszky, L.E. Novel male-specific molecular markers (MADC5, MADC6) in hemp. Euphytica 2002, 127, 209–218.
- Mandolino, G.; Ranalli, P. The Applications of Molecular Markers in Genetics and Breeding of Hemp. J. Ind. Hemp 2002, 7, 7–23.
- Moliterni, V.M.; Cattivelli, L.; Ranalli, P.; Mandolino, G. The sexual differentiation of Cannabis sativa L.: A morphological and molecular study. Euphytica 2004, 140, 95–106.
- Faux, A.-M.; Draye, X.; Flamand, M.-C.; Occre, A.; Bertin, P. Identification of QTLs for sex expression in dioecious and monoecious hemp (Cannabis sativa L.). Euphytica 2016, 209, 357–376.
- Heslop-Harrison, J.; Heslop-Harrison, Y. Studies on Flowering-Plant Growth and Organogenesis: III. Leaf Shape Changes Associated with Flowering and Sex Differentiation in Cannabis Sativa. In Proceedings of the Royal Irish Academy. Section B: Biological, Geological, and Chemical Science; Royal Irish Academy: Dublin, Ireland, 1957; Volume 59, pp. 257–283.
- Adal, A.M.; Doshi, K.; Holbrook, L.; Mahmoud, S.S. Comparative RNA-Seq analysis reveals genes associated with masculinization in female Cannabis sativa. Planta 2021, 253, 1–17.
- Samantaray, S.; Phurailatpam, A.; Bishoyi, A.K.; Geetha, K.A.; Maiti, S. Identification of sex-specific DNA markers in betel vine (Piper betle L.). Genet. Resour. Crop. Evol. 2011, 59, 645–653.
- Phurailatpam, A.K.; Geetha, K.A.; Maiti, S. Ploidy distinction in male and female plants of betelvine (Piper betle L.): A study by flow cytometry. Genet. Resour. Crop. Evol. 2018, 65, 1565–1570.
- Das, S.; Parida, R.; Sandeep, I.S.; Nayak, S.; Mohanty, S. Biotechnological intervention in betelvine (Piper betle L.): A review on recent advances and future prospects. Asian Pac. J. Trop. Med. 2016, 9, 938–946.
- Sheeja, T.E.; Bindu, K.H.; Anto, P.; Dhanya, K.; Siju, S.; Kumar, T.V. A SCAR marker based method for sex determination in dioecious betel vine (Piper betle). Ind. J. Agric. Sci. 2013, 83, 1409–1410.
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 25 October 2022).
- Denadi, N.; Gandonou, C.; Missihoun, A.A.; Zoundjihékpon, J.; Quinet, M. Plant Sex Prediction Using Genetic Markers in Cultivated Yams (Dioscorea rotundata Poir.) in Benin. Agronomy 2020, 10, 1521.
- Terauchi, R.; Kahl, G. Sex determination in Dioscorea tokoro, a wild yam species. In Sex Determination in Plants; Ainsworth, C.C., Ed.; BIOS Scientific Publishers: Oxford, UK, 1999; pp. 163–171.
- Martin, F.W. Sex Ratio and Sex Determination in Dioscorea. J. Hered. 1966, 57, 95–99.
- Tamiru, M.; Natsume, S.; Takagi, H.; White, B.; Yaegashi, H.; Shimizu, M.; Yoshida, K.; Uemura, A.; Oikawa, K.; Abe, A.; et al. Genome sequencing of the staple food crop white Guinea yam enables the development of a molecular marker for sex determination. BMC Biol. 2017, 15, 1–20.
- Sugihara, Y.; Darkwa, K.; Yaegashi, H.; Natsume, S.; Shimizu, M.; Abe, A.; Hirabuchi, A.; Ito, K.; Oikawa, K.; Tamiru-Oli, M.; et al. Genome analyses reveal the hybrid origin of the staple crop white Guinea yam (Dioscorea rotundata). Proc. Natl. Acad. Sci. USA 2020, 117, 31987–31992.
- Renner, S.S.; Feil, J.P. Pollinators of tropical dioecious angiosperms. Am. J. Bot. 1993, 80, 1100–1107.
- Liao, Z.; Yu, Q.; Ming, R. Development of male-specific markers and identification of sex reversal mutants in papaya. Euphytica 2017, 213, 1–12.
- Charlesworth, D. Young sex chromosomes in plants and animals. New Phytol. 2019, 224, 1095–1107.
- Yu, Q.; Hou, S.; Hobza, R.; Feltus, F.A.; Wang, X.; Jin, W.; Ming, R. Chromosomal location and gene paucity of the male specific region on papaya Y chromosome. Mol. Genet. Genom. 2007, 278, 177–185.
- Ming, R.; Yu, Q.; Moore, P.H. Sex determination in papaya. Semin. Cell Dev. Biol. 2007, 18, 401–408.
- Urasaki, N.; Tarora, K.; Shudo, A.; Ueno, H.; Tamaki, M.; Miyagi, N.; Adaniya, S.; Matsumura, H. Digital Transcriptome Analysis of Putative Sex-Determination Genes in Papaya (Carica papaya). PLoS ONE 2012, 7, e40904.
- Wang, J.; Na, J.-K.; Yu, Q.; Gschwend, A.R.; Han, J.; Zeng, F.; Aryal, R.; VanBuren, R.; Murray, J.E.; Zhang, W.; et al. Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13710–13715.
- Zhou, P.; Zhang, X.; Ma, X.; Yue, J.; Liao, Z.; Ming, R. Methylation related genes affect sex differentiation in dioecious and gynodioecious papaya. Hortic. Res. 2022, 9, uhab065.
- VanBuren, R.; Zeng, F.; Chen, C.; Zhang, J.; Wai, C.M.; Han, J.; Aryal, R.; Gschwend, A.R.; Wang, J.; Na, J.-K.; et al. Origin and domestication of papaya Yh chromosome. Genome Res. 2015, 25, 524–533.
- Lin, H.; Liao, Z.; Zhang, L.; Yu, Q. Transcriptome analysis of the male-to-hermaphrodite sex reversal induced by low temperature in papaya. Tree Genet. Genomes 2016, 12, 1–14.
- Ramos, H.C.C.; Pereira, M.G.; Da Silva, F.F.; Viana, A.P.; Ferreguetti, G.A. Seasonal and genetic influences on sex expression in a backcrossed segregating papaya population. Crop. Breed. Appl. Biotechnol. 2011, 11, 97–105.
- Martelleto, L.A.P.; Ribeiro, R.D.L.D.; Sudo-Martelleto, M.; Vasconcellos, M.A.D.S.; Pereira, M.B. Expressão da esterilidade feminina e da carpeloidia em mamoeiro sob diferentes ambientes de cultivo protegido. Rev. Bras. Frutic. 2011, 33, 1185–1193.
- This, P.; Lacombe, T.; Thomas, M.R. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006, 22, 511–519.
- Badouin, H.; Velt, A.; Gindraud, F.; Flutre, T.; Dumas, V.; Vautrin, S.; Marande, W.; Corbi, J.; Sallet, E.; Ganofsky, J.; et al. The wild grape genome sequence provides insights into the transition from dioecy to hermaphroditism during grape domestication. Genome Biol. 2020, 21, 1–24.
- Tamura, M.; Tao, R.; Yonemori, K.; Utsunomiya, N.; Sugiura, A. Ploidy Level and Genome Size of Several Diospyros Species. J. Jpn. Soc. Hortic. Sci. 1998, 67, 306–312.
- Akagi, T.; Kajita, K.; Kibe, T.; Morimura, H.; Tsujimoto, T.; Nishiyama, S.; Kawai, T.; Yamane, H.; Tao, R. Development of molecular markers associated with sexuality in Diospyros lotus L. and their application in D. kaki Thunb. J. Jpn. Soc. Hortic. Sci. 2013, 83, 214–221.
- Kafkas, S.; Açar, İ.; Gözel, H. A project on developing monoecious pistachio (Pistacia vera L.) populations and determination of sex mechanism in Pistacia. Options Méditérr 2003, 63, 57–60.
- Sheikhi, A.; Arab, M.M.; Brown, P.J.; Ferguson, L.; Akbari, M. Pistachio (Pistacia spp.) Breeding. In Advances in Plant Breeding Strategies: Nut and Beverage Crops; Springer: Cham, Switzerland, 2019; pp. 353–400.
- Sola-Campoy, P.J.; Robles, F.; Schwarzacher, T.; Rejón, C.R.; De La Herrán, R.; Navajas-Pérez, R. The Molecular Cytogenetic Characterization of Pistachio (Pistacia vera L.) Suggests the Arrest of Recombination in the Largest Heteropycnotic Pair HC1. PLoS ONE 2015, 10, e0143861.
- Kafkas, S.; Ma, X.; Zhang, X.; Topçu, H.; Navajas-Pérez, R.; Wai, C.M.; Tang, H.; Xu, X.; Khodaeiaminjan, M.; Güney, M.; et al. Pistachio genomes provide insights into nut tree domestication and ZW sex chromosome evolution. Plant Commun. 2022, in press.
- Puterova, J.; Razumova, O.; Martinek, T.; Alexandrov, O.; Divashuk, M.; Kubat, Z.; Hobza, R.; Karlov, G.; Kejnovsky, E. Satellite DNA and Transposable Elements in Seabuckthorn (Hippophae rhamnoides), a Dioecious Plant with Small Y and Large X Chromosomes. Genome Biol. Evol. 2017, 9, 197–212.
- Yu, L.; Diao, S.; Zhang, G.; Yu, J.; Zhang, T.; Luo, H.; Duan, A.; Wang, J.; He, C.; Zhang, J. Genome sequence and population genomics provide insights into chromosomal evolution and phytochemical innovation of Hippophae rhamnoides. Plant Biotechnol. J. 2022, 20, 1257–1273.
- Luo, X.; Liu, J.; He, Z. Oligo-FISH Can Identify Chromosomes and Distinguish Hippophaë rhamnoides L. Taxa. Genes 2022, 13, 195.
- Sharma, A.; Zinta, G.; Rana, S.; Shirko, P. Molecular identification of sex in Hippophae rhamnoides L. using isozyme and RAPD markers. For. Stud. China 2010, 12, 62–66.
- Persson, H.A.; Nybom, H. Genetic Sex Determination and RAPD Marker Segregation in the Dioecious Species Sea Buckthorn (Hippophae Rhamnoides L.). Hereditas 2004, 129, 45–51.
- Zhou, W.; Wang, Y.; Zhang, G.; Luan, G.; Chen, S.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Molecular Sex Identification in Dioecious Hippophae rhamnoides L. via RAPD and SCAR Markers. Molecules 2018, 23, 1048.
- Stobdan, T.; Mishra, G.P.; Yadav, A.; Chaurasia, O.P. Methods in Seabuckthorn Breeding. In The Seabuckthorn Genome; Springer: Cham, Switzerland, 2022; pp. 331–344.
- Divashuk, M.; Alexandrov, O.; Kroupin, P.; Karlov, G. Molecular Cytogenetic Mapping of Humulus lupulus Sex Chromosomes. Cytogenet. Genome Res. 2011, 134, 213–219.
- Karlov, G.; Danilova, T.; Horlemann, C.; Weber, G. Molecular cytogenetics in hop (Humulus lupulus L.) and identification of sex chromosomes by DAPI-banding. Euphytica 2003, 132, 185–190.
- Neve, R.A. Sex Chromosomes in the Hop Humulus lupulus. Nature 1958, 181, 1084–1085.
- Neve R., A. Hops; Chapman and Hall: London, UK, 1991.
- Čerenak, A.; Kolenc, Z.; Sehur, P.; Whittock, S.P.; Koutoulis, A.; Beatson, R.; Buck, E.; Javornik, B.; Škof, S.; Jakše, J. New Male Specific Markers for Hop and Application in Breeding Program. Sci. Rep. 2019, 9, 14223.
- Danilova, T.V.; Karlov, G.I. Application of inter simple sequence repeat (ISSR) polymorphism for detection of sex-specific molecular markers in hop (Humulus lupulus L.). Euphytica 2006, 151, 15–21.
- Polley, A.; Ganal, M.W.; Seigner, E. Identification of sex in hop (Humulus lupulus) using molecular markers. Genome 1997, 40, 357–361.
- Jakše, J.; Štajner, N.; Kozjak, P.; Čerenak, A.; Javornik, B. Trinucleotide microsatellite repeat is tightly linked to male sex in hop (Humulus lupulus L.). Mol. Breed. 2007, 21, 139–148.
- Haunold, A. Cytology, Sex Expression, and Growth of a Tetraploid ✕ Diploid Cross in Hop (Humulus lupulus L.) 1. Crop. Sci. 1971, 11, 868–871.
- Beatson, R.A.; Ferguson, A.R.; Weir, I.E.; Graham, L.T.; Ansell, K.A.; Ding, H. Flow cytometric identification of sexually derived polyploids in hop (Humulus lupulus L.) and their use in hop breeding. Euphytica 2003, 134, 189–194.
- Younis, R.A.; Ismail, O.M.; Soliman, S.S. Identification of sex-specific DNA markers for date palm (Phoenix dactylifera L.) using RAPD and ISSR techniques. Res. J. Agric. Biol. Sci. 2008, 4, 278–284.
- Jaskani, M.J.; Awan, F.S.; Ahmad, S.; Khan, I.A. Maryam Development of molecular method for sex identification in date palm (Phoenix dactylifera L.) plantlets using novel sex-linked microsatellite markers. 3 Biotech 2016, 6, 1–7.
- Intha, N.; Chaiprasart, P. Sex determination in date palm (Phoenix dactylifera L.) by PCR based marker analysis. Sci. Hortic. 2018, 236, 251–255.
- Elmeer, K.; Mattat, I. Marker-assisted sex differentiation in date palm using simple sequence repeats. 3 Biotech 2012, 2, 241–247.
- Dhawan, C.; Kharb, P.; Sharma, R.; Uppal, S.; Aggarwal, R.K. Development of male-specific SCAR marker in date palm (Phoenix dactylifera L.). Tree Genet. Genomes 2013, 9, 1143–1150.
- Torres, M.F.; Mathew, L.S.; Ahmed, I.; Al-Azwani, I.K.; Krueger, R.; Rivera-Nuñez, D.; Mohamoud, Y.A.; Clark, A.G.; Suhre, K.; Malek, J.A. Genus-wide sequencing supports a two-locus model for sex-determination in Phoenix. Nat. Commun. 2018, 9, 1–9.
- Nair, R.R. Chromosome number analysis in different sex types and open-pollinated seedlings of nutmeg (Myristica fragrans Houtt). J. Plant. Crops 2019, 47, 197–201.
- Olajide, O.A.; Ajayi, F.F.; Ekhelar, A.I.; Awe, S.O.; Makinde, J.M.; Alada, A.A. Biological effects of Myristica fragrans (nutmeg) extract. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 1999, 13, 344–345.
- Shaanker, U. ldentification of sex-specific DNA markers in the dioecious tree, nutmeg (Myristica fragrans Houtt.). Noticiario de Recursos Fitogenéticos 2000.
- Mintah, F.D. Sex Determination in Nutmeg Seedlings Using Scar Primers. J. Hortic. Plant Res. 2018, 3, 40–47.
- Nikam, D.P.; Ingale, P.C.; Gokhale, N.B.; Lajurkar, V.G. Sex Determination in Nutmeg (Myristica fragrance Hott.) by using RAPD Markers. Indian Hortic. J. 2016, 6, 148–149.
- Mcneilage, M.A. Gender variation in Actinidia deliciosa, the kiwifruit. Sex. Plant Reprod. 1991, 4, 267–273.
- Ferguson, A.R. Kiwifruit: The wild and the cultivated plants. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2013; Volume 68, pp. 15–32.
- Ferguson, A.R.; Huang, H. Genetic resources of kiwifruit: Domestication and breeding. Hortic. Rev. 2007, 33, 1–121.
- Yan, G.; Yao, J.; Ferguson, A.R.; Mcneilage, M.A.; Seal, A.G.; Murray, B.G. New reports of chromosome numbers in Actinidia (Actinidiaceae). N. Z. J. Bot. 1997, 35, 181–186.
- Gill, G.P.; Harvey, C.F.; Gardner, R.C.; Fraser, L.G. Development of sex-linked PCR markers for gender identification in Actinidia. Theor. Appl. Genet. 1998, 97, 439–445.
- Seal, A.G.; Ferguson, A.R.; De Silva, H.N.; Zhang, J.-L. The effect of 2n gametes on sex ratios in Actinidia. Sex. Plant Reprod. 2012, 25, 197–203.
- Fraser, L.G.; Tsang, G.K.; Datson, P.M.; De Silva, H.N.; Harvey, C.F.; Gill, G.P.; Crowhurst, R.N.; Mcneilage, M.A. A gene-rich linkage map in the dioecious species Actinidia chinensis (kiwifruit) reveals putative X/Y sex-determining chromosomes. BMC Genom. 2009, 10, 102.
- He, Z.-C.; Li, J.Q.; Cai, Q.; Wang, Q. The cytology of Actinidia, Saurauia and Clematoclethra (Actinidiaceae). Bot. J. Linn. Soc. 2005, 147, 369–374.
- Akagi, T.; Henry, I.M.; Ohtani, H.; Morimoto, T.; Beppu, K.; Kataoka, I.; Tao, R. A Y-Encoded Suppressor of Feminization Arose via Lineage-Specific Duplication of a Cytokinin Response Regulator in Kiwifruit. Plant Cell 2018, 30, 780–795.
- Zhang, Q.; Liu, C.; Liu, Y.; VanBuren, R.; Yao, X.; Zhong, C.; Huang, H. High-density interspecific genetic maps of kiwifruit and the identification of sex-specific markers. DNA Res. 2015, 22, 367–375.
- Shirkot, P.; Sharma, D.R.; Mohapatra, T. Molecular identification of sex in Actinidia deliciosa var. deliciosa by RAPD markers. Sci. Hortic. 2002, 94, 33–39.
- Hale, I.; Melo, A.; Gustafson, H. Sex-linked molecular markers for two cold-hardy kiwifruit species, Actinidia arguta and A. kolomikta. Eur. J. Hortic. Sci. 2018, 83, 236–246.
- Chłosta, I.; Kwolek, D.; Sliwinska, E.; Góralski, G.; Popielarska-Konieczna, M. Sex-Linked Molecular Markers Identify Female Lines in Endosperm-Derived Kiwifruit Callus and in Regenerants. Plants 2021, 10, 526.
- Rakocevic, M.; Medrado, M.; Martim, S.; Assad, E. Sexual dimorphism and seasonal changes of leaf gas exchange in the dioecious tree Ilex paraguariensis grown in two contrasted cultivation types. Ann. Appl. Biol. 2009, 154, 291–301.
- Gaiad, S.; Rakocevic, M.; Reissmann, C.B. N sources affect growth, nutrient content, and net photosynthesis in maté (Ilex paraguariensis St. Hil.). Braz. Arch. Biol. Technol. 2006, 49, 689–697.
- Rakocevic, M.; Costes, E.; Assad, E. Structural and physiological sexual dimorphism estimated from three-dimensional virtual trees of yerba-mate (Ilex paraguariensis) is modified by cultivation environment. Ann. Appl. Biol. 2011, 159, 178–191.
- Rakocevic, M.; Medrado, M.J.S. Quality of yerba-mate leaves originating from male and female plants. Pesqui. Florest. Bras. 2007, 54, 71–83.
- Gottlieb, A.M.; Poggio, L. Genomic screening in dioecious “yerba mate” tree (Ilex paraguariensis A. St. Hill., Aquifoliaceae) through representational difference analysis. Genetica 2010, 138, 567–578.
- Golan-Goldhirsch, A.; Jones, R.; Rowland, L. AFLP markers for sex determination in an ilex species. Acta Hortic. 2001, 546, 591–595.
- Gauer, L.; Cavalli-Molina, S. Genetic variation in natural populations of maté (Ilex paraguariensis A. St.-Hil., Aquifoliaceae) using RAPD markers. Heredity 2000, 84, 647–656 .
- Torimaru, T.; Tani, N.; Tsumura, Y.; Hiraoka, K.; Tomaru, N. Development and polymorphism of simple sequence repeat DNA markers for the evergreen shrub Ilex leucoclada M. Mol. Ecol. Notes 2004, 4, 531–533.
- Chen, K.; Xu, C. Red bayberry: Botany and horticulture. In Horticultural Reviews; John Wiley & Sons Inc.: Oxford, UK, 2010; pp. 83–114.
- Stokes, J. Cytological Studies in the Myricaceae. Bot. Gaz. 1937, 99, 387–399.
- Wang, Y.; Jia, H.-M.; Shen, Y.-T.; Zhao, H.-B.; Yang, Q.-S.; Zhu, C.-Q.; Sun, D.-L.; Wang, G.-Y.; Zhou, C.-C.; Jiao, Y.; et al. Construction of an anchoring SSR marker genetic linkage map and detection of a sex-linked region in two dioecious populations of red bayberry. Hortic. Res. 2020, 7, 1–9.
- Jia, H.; Zhao, L.; Wang, Y.; Wu, H.; Zhao, H.; Zhu, Y.; Jiao, Y.; Wang, G.; Zhou, C.; Huang, C.; et al. Comparative Transcriptome Analysis Reveals Sex-Biased Expression of Hormone-Related Genes at an Early Stage of Sex Differentiation in Red Bayberry (Morella rubra). Horticulturae 2022, 8, 183.
- Li, T.S.; Schroeder, W. Sea Buckthorn (Hippophae rhamnoides L.): A Multipurpose Plant. Horttechnology 1996, 6, 370–380.




