
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kai-hui Nan | -- | 2304 | 2023-02-17 10:34:28 | | | |
| 2 | Kai-hui Nan | Meta information modification | 2304 | 2023-02-17 10:36:30 | | | | |
| 3 | Rita Xu | Meta information modification | 2304 | 2023-02-17 10:48:28 | | | | |
| 4 | Kai-hui Nan | -1618 word(s) | 686 | 2023-02-21 02:17:41 | | | | |
| 5 | Kai-hui Nan | + 1901 word(s) | 2587 | 2023-02-22 07:06:50 | | | | |
| 6 | Rita Xu | Meta information modification | 2587 | 2023-02-22 07:15:09 | | |
Video Upload Options
Dry eye disease (DED) is a widespread and frequently reported multifactorial ocular disease that not only causes ocular discomfort but also damages the cornea and conjunctiva. Topical administration is the most common treatment modality for DED. Due to the existence of multiple biological barriers, instilled drugs generally exhibit short action times and poor penetration on the ocular surface. To resolve these issues, several advanced drug delivery systems have been proposed.
1. Introduction
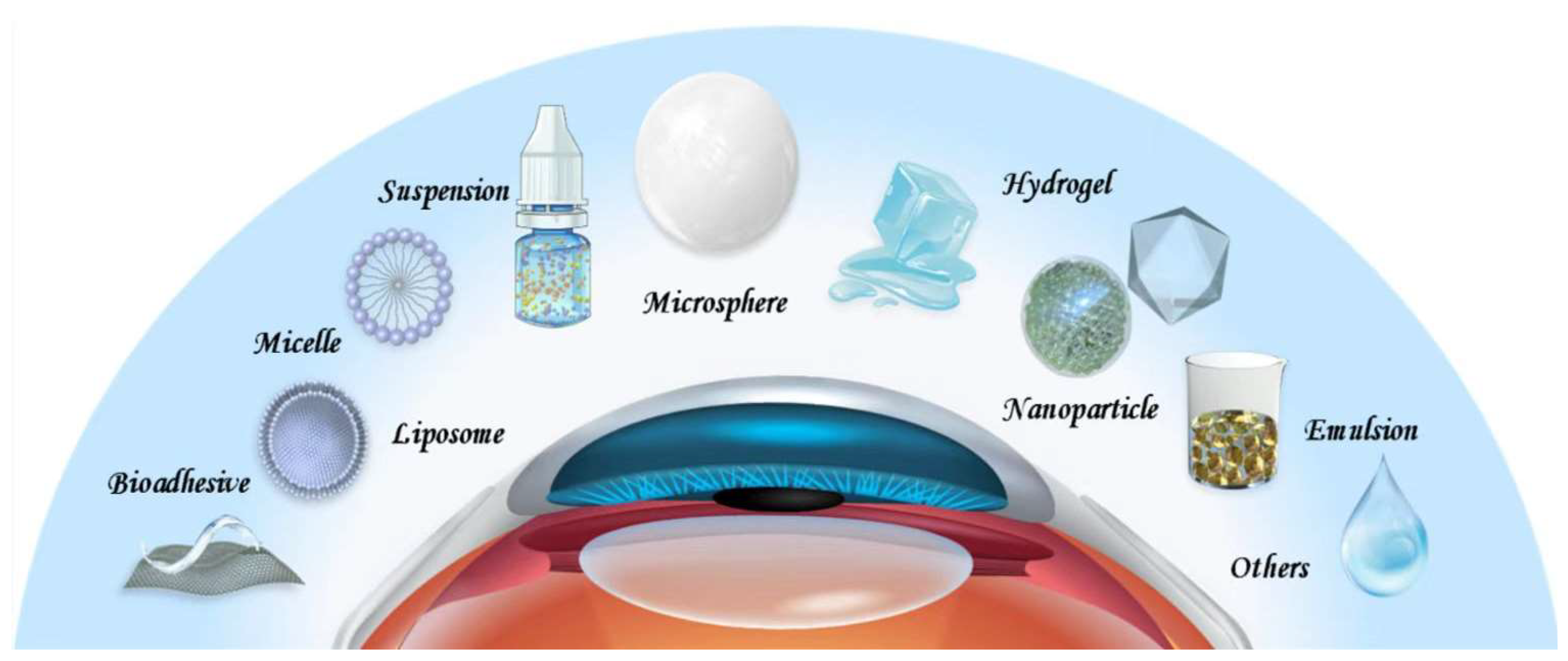
2. Drug Delivery Systems for DED
2.1. Suspensions
Suspension refers to a liquid formulation formed by dispersing insoluble drug particles in a liquid medium. Common methods for the preparation of suspension include direct dispersion, precipitation, and controlled flocculation. Drug particles in the suspension settle slowly at a rate that does not interfere with correct dosing. They do not agglomerate and can be dispersed evenly through shaking, even after long-term storage. After topical application, drug particles in the suspension can be retained in the cul-de-sac, which leads to prolonged ocular action duration [28]. The hydrophobic drugs currently used for the treatment of DED are often administered in this manner.
Alrex® (Bausch & Lomb, Clearwater, FL, USA) is an FDA-approved loteprednol etabonate suspension primarily used as an anti-inflammatory agent. Clinical trials have confirmed that DED can be treated effectively by monotherapy with this drug or combination therapy containing artificial tears [29][30]. Furthermore, suspensions of the immunosuppressant cyclosporine and the mucin-stimulating drug rebamipide have also been approved for treating DED [22][31]. In a randomized multicenter phase III study, 2% rebamipide suspension and 0.1% sodium hyaluronate were randomly instilled in 188 patients with DED, which demonstrated that the 2% rebamipide suspension was more effective in relieving foreign body sensation as well as eye pain [32].
Although rebamipide suspension is advantageous for the treatment of DED, it is a milky liquid and blurs the patient’s vision temporarily, which reduces visual quality. To overcome this shortcoming, Matsuda et al. [33] formulated rebamipide particles into an ultrafine state (approximately 640 nm in size) to obtain a highly transparent (light transmittance: 59%) suspension. The duration of blurred vision was thus reduced. Moreover, an in vivo pharmacokinetics study revealed that the concentrations of rebamipide in cornea and conjunctiva were higher than those of conventional suspension, which indicated accelerated absorption rates and improved bioavailability. The particle size and transparency of this suspension remain unchanged for 3 years when stored at 25 °C, which demonstrated its excellent physicochemical stability. Augmenting the mucus-penetrating abilities of drugs represents another strategy to obtain improved outcomes. In this regard, Eysuvis® (Kala Pharmaceuticals, Arlington, MA, USA) is an original loteprednol nanosuspension developed by Kala using the AMPPLIFY mucus-penetrating particle drug delivery technology. This technology permits loteprednol to reach the ocular surface without being degraded in the tear film. A single application of Eysuvis® can increase the concentrations of loteprednol in the aqueous humor, cornea, and conjunctiva by up to three times compared with the commercial product Lotemax® (0.38% loteprednol etabonate eye gel, Bausch & Lomb, Clearwater, FL, USA). Eysuvis® showed high efficacy in DED treatment [34][35]. Moreover, a multicenter randomized clinical trial demonstrated it was safe and well-tolerated for short-term use (2–4 weeks) [36]. However, the long-term in vivo safety of it remains to be determined.
2.2. Emulsions
Emulsion refers to a two-phase liquid in which the two phases are immiscible with each other. It generally consists of an aqueous phase (denoted by W), an oil phase (denoted by O), and an emulsifier. One phase is dispersed in the other in the form of small droplets, leading to a heterogeneous dispersion. The oil-in-water (O/W) emulsion serves as a good delivery vehicle for hydrophobic ophthalmic drugs, because the oil phase of it can be harnessed to dissolve poorly water-soluble molecules. As a result, improved ocular bioavailability is obtained [37]. Restasis® (Allergan, Waco, TX, USA), an O/W anionic nanoemulsion containing cyclosporine, is the first commercially available ophthalmic emulsion for DED. It is obtained by dissolving cyclosporine in castor oil using polysorbate as the emulsifier. The drug droplets of Restasis® spread easily on the ocular surface after instillation, thus allowing fast drug absorption and onset of action [38]. Emulsions also increase the solubility of hydrophobic drugs and offer ease of scale production in industrial settings [39].
Ikervis® is an O/W cationic nanoemulsion of cyclosporine developed by Santen Pharmaceutical (Osaka, Japan). The drug concentration of Ikervis® is the highest in the clinic. It is used in patients with severe DED that cannot be ameliorated by artificial tears. Because the emulsion droplets in Ikervis® are smaller than those in Restasis®, the penetration of cyclosporine into the cornea is enhanced. Moreover, the unique cationic Ikervis® can interact electrostatically with the negatively charged ocular surface, which prolongs residence time of the drug. The dosing frequency of Ikervis® is one time per day. This has been associated with improved patient compliance and therapeutic efficiency. A good safety profile of Ikervis® had also been demonstrated in a 12-month multicenter double-blind clinical trial [40]. Cationic emulsions are prone to cause local side effects, such as mild pain at the instillation site [41][42]. To improve eye comfort and bioavailability of the drug, Bang et al. developed self-emulsifying nanodrug delivery systems, namely, Cyporin-N (SNEDDS-N; Taejoon Pharma, Seoul, Republic of Korea) and T-sporin (SNEDDS-T; Taejoon Pharma, Seoul, Republic of Korea). SNEDDS is an anhydrous homogeneous mixture of oils, drugs, surfactants, and cosurfactants. Compared with the high turbidity and unstable pH of Restasis®, SNEDDS exhibits more uniform particle sizes, better light transmission, and more stable pH. The ability of SNEDDS to restore tear film stability is also superior to that of Restasis® [43]. Currently, SNEDDS has been developed in a variety of forms and diseases, such as solid SNEDDS, controlled-release SNEDDS, mucus-permeable SNEDDS, and targeted SNEDDS [44][45][46]. It is hoped that these innovative formulations will lead to a breakthrough in the management of DED. Apart from drug-loaded emulsions, drug-free emulsions have also been developed for DED. For instance, Cationorm® from Santen Pharmaceutical is a drug-free O/W cationic nanoemulsion. The benzylcetyldimethylammonium chloride presented in Cationorm® is a cationic surfactant that possesses intrinsic antimicrobial activity, which makes Cationorm® a preservative-free dosage form. This design enhances the safety of Cationorm®. Pharmacodynamic studies revealed that Cationorm® not only exhibited moisturizing and lubricating properties but also stabilized the tear film by its oily components, thus making it a safe and effective tear supplement [47][48].
2.3. Liposomes
Liposomes are tiny vesicles of 10–1000 nm which are consisted of natural or synthetic phospholipid bilayers. They can be classified as small unilamellar vesicles, large unilamellar vesicles, giant unilamellar vesicles, oligolamellar vesicles, multilamellar large vesicles, and multivesicular vesicles [49]. Film hydration, reverse phase evaporation, solvent injection, detergent removal, and the heating method are conventionally used for the preparation of liposomes. Meanwhile, new technologies such as microfluidic methods and the supercritical fluidic method have gained considerable attention [50]. Liposomes improve the delivery of ophthalmic drugs by encapsulating hydrophobic ones in phospholipid bilayers or encapsulating hydrophilic ones in the aqueous core. With similar structure and composition as the cell membrane, liposomes are generally biodegradable and well-tolerated [51][52][53].
The Tears Again® liposome spray developed by Optima Pharmazeutische (Hallbergmoos, Germany) is a new generation of liposome supplements capable of repairing all three layers of the tear film. The phospholipids in the ingredients repair the lipid layer of the tear film, the isotonic solution replenishes the aqueous layer, and the sodium hyaluronate serves as an alternative to the mucus layer. Upon application, the active ingredients are absorbed transdermally. Meanwhile, the preservatives are blocked outside the skin and do not enter the tear film, thereby preventing damage to the eyes. In a double-blind clinical trial, the Tears Again® liposome spray was found to reduce discomfort and stabilize the tear film more efficiently than saline spray and 0.1% sodium hyaluronate [54][55]. In another example, Chen et al. prepared tacrolimus-loaded cationic liposomes using the film hydration method. The resultant formulation prolongs the retention time of FK506 on the ocular surface, increases its concentration in the cornea, and exerts a good therapeutic effect owing to its anti-inflammatory property and ability to promote epithelial cell healing [56]. Ren T et al. formulated adriamycin ion pair loaded liposomes to improve the therapeutic effects of the drug against DED. First, an adriamycin–cholesterol hemisuccinate ion pair was prepared to improve the drug loading. Second, liposomes were prepared by a film hydration method. Finally, the liposomes were sonicated to obtain uniform particle size with high drug loading efficiency [57]. Recently, the macromolecular protein lactoferrin and the antioxidant astaxanthin have also been encapsulated in liposomes, which showed favorable therapeutic effects against DED as proved by in vivo pharmacodynamics studies [58][59][60].
2.4. Nanoparticles
Nanoparticles for drug encapsulation are nanosized delivery vehicles with particle sizes in the range of 1–1000 nm. The majority of them in ophthalmic applications are made from natural or synthetic polymers (gelatin, silk fibroin, chitosan, PLGA, PLA, PCL, etc.). Various methods, such as nanoprecipitation, self-assembly, ionic gelation, and desolvation, have been developed for their preparation. Superior therapeutic effects against ocular diseases are obtained via different mechanisms including increasing the dissolution/solubility of poorly water-soluble drugs, affording sustained release, enhancing ocular retention/penetration, or transporting drugs to targeted tissues/cells [35].
The residence time of eye drops is short due to blink and nasolacrimal duct drainage, which lead to low drug bioavailability. To overcome this drawback, Nagai et al. fabricated a rebamipide-based solid nanoparticle formulation (REB-NPs) via grinding. The thus-obtained rebamipide nanoparticles were elliptical, with particle sizes in the range of 40–200 nm. After being applied to the eyelid, rebamipide can be delivered to the tear film through meibomian glands. The rebamipide nanoparticles prolonged the release duration of rebamipide compared with the conventional rebamipide suspension. Thus, increased mucin levels and tear film restoration were obtained [60]. As the vicious cycle of DED is generally accompanied with a series of changes in the ocular surface microenvironment, nanoparticles with dual or multiple functions are likely to produce synergistic effects and lead to improved therapeutic outcomes. Li YJ et al. developed an AF/Au@Poly-CH nanoparticle formulation with anti-inflammatory and antioxidant functions. AF/Au@Poly-CH was obtained via a one-step self-assembly process. In vivo safety and efficacy studies in rabbit eyes revealed that the resultant nanoformulation was well-tolerated and demonstrated high therapeutic efficacy [62].
Drug-loaded nanoparticles have also been formulated into dosage forms other than eye drops. For example, Ryu et al. developed nanoparticles incorporated tablets, by embedding PLGA nanoparticles containing dexamethasone in an alginate matrix. The table was applied to the ocular surface using a preocular applicator. It was found that the nanoparticles remained on the ocular surface for up to 2 h. This mode of administration not only improves the bioavailability of drugs but also enables their sterile delivery [63][64]. Nanocapsules are nanoparticles with hollow cores, which are mainly used to deliver labile drugs or engineered for targeted delivery. Although several nanocapsule-based delivery systems exist for anti-inflammatory drugs, few reports are available with respect to their application for the treatment of DED [65][66]. Zhang et al. prepared cyclosporine lipid nanocapsule eye drops with the phase-inversion method, which increased bioavailabilities of cyclosporine in the conjunctiva and cornea. In line with the pharmacokinetic study, superior therapeutic effects over conventional cyclosporine emulsion were observed in pharmacodynamic studies [67].
3. Conclusions
Other drug delivery systems, such as microspheres, hydrogels, and bioadhesive polymers have also been engineered to improve therapeutic effects of DED drugs. Indeed, promising results are obtained, which have the potential to lead to innovative therapies. Considering the shortcomings of each drug delivery system, the combination of two or more of them deserves further research. Current treatments for DED generally target one aspect of DED pathophysiology. It is fascinating to explore whether superior outcomes can be obtained with drug delivery systems that target multiple aspects simultaneously. There is also a lack of biodegradability and in vivo safety information concerning the above-mentioned drug delivery systems, as most studies are conducted in the short-term setting. A direct comparison between these delivery vehicles represents another issue to be addressed to determine the most suitable one. Finding answers to these questions constitutes the key areas of future research to improve drug delivery for DED.
References
- Hakim, F.; Farooq, A. Dry Eye Disease: An Update in 2022. JAMA 2022, 327, 478–479.
- Messmer, E. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81.
- Tsubota, K.; Pflugfelder, S.; Liu, Z.; Baudouin, C.; Kim, H.; Messmer, E.; Kruse, F.; Liang, L.; Carreno-Galeano, J.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271.
- Han, K.-T.; Nam, J.H.; Park, E.-C. Do Sleep Disorders Positively Correlate with Dry Eye Syndrome? Results of National Claim Data. Int. J. Environ. Res. Public Health 2019, 16, 878.
- Schaumberg, D.; Dana, R.; Buring, J.; Sullivan, D. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768.
- Lee, J.-H.; Lee, W.; Yoon, J.-H.; Seok, H.; Roh, J.; Won, J.-U. Relationship between symptoms of dry eye syndrome and occupational characteristics: The Korean National Health and Nutrition Examination Survey 2010–2012. BMC Ophthalmol. 2015, 15, 147.
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and Risk Factors of Dry Eye Disease in Japan: Koumi Study. Ophthalmology 2011, 118, 2361–2367.
- Maychuk, D.Y.; Anisimova, S.; Kapkova, S.; Kachanov, A.; Korotkikh, S.; Seleznev, A.; Sakhnov, S.; Leonova, E.; Krylov, S. Prevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in Russia. J. Cataract Refract. Surg. 2016, 42, 427–434.
- Wu, H.-X.; Xia, X.; Liu, K.; Zheng, Z.; Zhu, N.-Q.; Xu, X.; Gu, Q. Effect of insulin on VEGF expression in bovine retinal microvascular endothelial cells exposed to normal or high glucose. Chin. J. Ophthalmol. 2008, 44, 640–644.
- Li, J.; Zheng, K.; Deng, Z.; Zheng, J.; Ma, H.; Sun, L.; Chen, W. Prevalence and Risk Factors of Dry Eye Disease Among a Hospital-Based Population in Southeast China. Eye Contact Lens Sci. Clin. Pract. 2015, 41, 44–50.
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365.
- Wang, M.T.M.; Craig, J.P. Core Outcome Sets for Clinical Trials in Dry Eye Disease. JAMA Ophthalmol. 2018, 136, 1180–1181.
- O’Neil, E.C.; Henderson, M.; Massaro-Giordano, M.; Bunya, V.Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 2019, 30, 166–178.
- Thulasi, P.; Djalilian, A.R. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology 2017, 124, S27–S33.
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41.
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457.
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223.
- Nasir, N.A.A.; Agarwal, P.; Agarwal, R.; Iezhitsa, I.; Alyautdin, R.; Nukolova, N.N.; Chekhonin, V.P.; Ismail, N.M. Intraocular distribution of topically applied hydrophilic and lipophilic substances in rat eyes. Drug Deliv. 2016, 23, 2765–2771.
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423.
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Singh, T.R.R. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889.
- Nosch, D.S.; Joos, R.E.; Job, M. Prospective randomized study to evaluate the efficacy and tolerability of Ectoin® containing Eye Spray (EES09) and comparison to the liposomal Eye Spray Tears Again® (TA) in the treatment of dry eye disease. Contact Lens Anterior Eye 2021, 44, 101318.
- Pflugfelder, S.; de Paiva, C. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13.
- Şimşek, C.; Doğru, M.; Kojima, T.; Tsubota, K. Current Management and Treatment of Dry Eye Disease. Turk. J. Ophthalmol. 2018, 48, 309–313.
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior Eye Segment Drug Delivery Systems: Current Treatments and Future Challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105.
- Yellepeddi, V.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82.
- Baino, F.; Kargozar, S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering 2020, 7, 65.
- de Paiva, C.; Pflugfelder, S.; Ng, S.; Akpek, E. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst. Rev. 2019, 9, Cd010.
- Bron, A.; de Paiva, C.; Chauhan, S.; Bonini, S.; Gabison, E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510.
- Beckman, K.; Katz, J.; Majmudar, P.; Rostov, A. Loteprednol Etabonate for the Treatment of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2020, 36, 497–511.
- Wan, P.-X.; Wang, X.-R.; Song, Y.-Y.; Li, Z.-Y.; Duan, H.-C.; Zhang, W.; Liu, Z.; Wang, Z.-C. Study on the treatment of dry eye with Loteprednol Etabonate. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2012, 48, 142–147.
- Kashima, T.; Akiyama, H.; Kishi, S.; Itakura, H. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: A critical appraisal. Clin. Ophthalmol. 2014, 8, 1003–1010.
- Kinoshita, S.; Oshiden, K.; Awamura, S.; Suzuki, H.; Nakamichi, N.; Yokoi, N. A Randomized, Multicenter Phase 3 Study Comparing 2% Rebamipide (OPC-12759) with 0.1% Sodium Hyaluronate in the Treatment of Dry Eye. Ophthalmology 2013, 120, 1158–1165.
- Matsuda, T.; Hiraoka, S.; Urashima, H.; Ogura, A.; Ishida, T. Preparation of an Ultrafine Rebamipide Ophthalmic Suspension with High Transparency. Biol. Pharm. Bull. 2017, 40, 665–674.
- Paton, D. Loteprednol etabonate: A formulation for short-term use in inflammatory flares in dry eye disease. Drugs Today 2022, 58, 77.
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538.
- Korenfeld, M.; Nichols, K.K.O.; Goldberg, D.; Evans, D.O.; Sall, K.; Foulks, G.; Coultas, S.; Brazzell, K. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients with Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea 2021, 40, 564–570.
- Yamaguchi, M.; Yasueda, S.-I.; Isowaki, A.; Yamamoto, M.; Kimura, M.; Inada, K.; Ohtori, A. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int. J. Pharm. 2005, 301, 121–128.
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318.
- Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185.
- Baudouin, C.; De La Maza, M.S.; Amrane, M.; Garrigue, J.-S.; Ismail, D.; Figueiredo, F.C.; Leonardi, A. One-Year Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease. Eur. J. Ophthalmol. 2017, 27, 678–685.
- Leonardi, A.; Van Setten, G.; Amrane, M.; Ismail, D.; Garrigue, J.-S.; Figueiredo, F.C.; Baudouin, C. Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease: A Multicenter Randomized Trial. Eur. J. Ophthalmol. 2016, 26, 287–296.
- Hoy, S.M. Ciclosporin Ophthalmic Emulsion 0.1%: A Review in Severe Dry Eye Disease. Drugs 2017, 77, 1909–1916.
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805.
- Nazlı, H.; Mesut, B.; Özsoy, Y. In Vitro Evaluation of a Solid Supersaturated Self Nanoemulsifying Drug Delivery System (Super-SNEDDS) of Aprepitant for Enhanced Solubility. Pharmaceuticals 2021, 14, 1089.
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703.
- Arshad, R.; Tabish, T.; Kiani, M.; Ibrahim, I.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086.
- Amrane, M.; Creuzot-Garcher, C.; Robert, P.-Y.; Ismail, D.; Garrigue, J.-S.; Pisella, P.-J.; Baudouin, C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease—A randomised comparative study. J. Fr. Ophtalmol. 2014, 37, 589–598.
- Lyseng-Williamson, K.A. Cationorm® (cationic emulsion eye drops) in dry eye disease: A guide to its use. Drugs Ther. Perspect. 2016, 32, 317–322.
- Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W.M. General Perception of Liposomes: Formation, Manufacturing and Applications. In Liposomes-Advances and Perspectives; IntechOpen: Rijeka, Croatia, 2019.
- Liang, W.; Levchenko, T.S.; Torchilin, V.P. Encapsulation of ATP into liposomes by different methods: Optimization of the procedure. J. Microencapsul. 2004, 21, 251–261.
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.
- López-Cano, J.; González-Cela-Casamayor, M.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847.
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Nasir, N.A.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091.
- Craig, J.P.; Purslow, C.; Murphy, P.J.; Wolffsohn, J.S. Effect of a liposomal spray on the pre-ocular tear film. Contact Lens Anterior Eye 2010, 33, 83–87.
- Khaireddin, R.; Schmidt, K. Comparative investigation of treatments for evaporative dry eye. Klin. Mon. Augenheilkd. 2010, 227, 128–134.
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168.
- Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol. Pharm. 2018, 15, 4862–4871.
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698.
- Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye disease model. J. Clin. Biochem. Nutr. 2020, 66, 224–232.
- Shimokawa, T.; Yoshida, M.; Fukuta, T.; Tanaka, T.; Inagi, T.; Kogure, K. Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. J. Clin. Biochem. Nutr. 2019, 64, 27–35.
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155.
- Li, Y.-J.; Luo, L.-J.; Harroun, S.G.; Wei, S.-C.; Unnikrishnan, B.; Chang, H.-T.; Huang, Y.-F.; Lai, J.-Y.; Huang, C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale 2019, 11, 5580–5594.
- Ryu, W.M.; Kim, S.-N.; Min, C.H.; Bin Choy, Y. Dry Tablet Formulation of PLGA Nanoparticles with a Preocular Applicator for Topical Drug Delivery to the Eye. Pharmaceutics 2019, 11, 651.
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control Release 2015, 213, 168–174.
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical tacrolimus nanocapsules eye drops for therapeutic effect enhancement in both anterior and posterior ocular inflammation models. J. Control Release 2021, 333, 283–297.
- Hu, Q.; Wu, W.; Wang, M.; Shao, S.; Jin, P.; Chen, Q.; Bai, H.; Zhao, X.; Huang, J.; Wang, J.; et al. Reverting chemoresistance of targeted agents by a ultrasoluble dendritic nanocapsule. J. Control Release 2020, 317, 67–77.
- Zhang, R.; Sun, M.; Ran, Y.; Deng, Y.; Ge, Y.; Zhu, X.; Tao, L.; Shang, J.; Gou, H.; He, T.; et al. A Novel Eyes Topical Drug Delivery System: CsA-LNC for the Treatment of DED. Pharm. Res. 2020, 37, 146.




