Dry eye disease (DED) is a widespread and frequently reported multifactorial ocular disease that not only causes ocular discomfort but also damages the cornea and conjunctiva. Topical administration is the most common treatment modality for DED. Due to the existence of multiple biological barriers, instilled drugs generally exhibit short action times and poor penetration on the ocular surface. To resolve these issues, several advanced drug delivery systems have been proposed.
Dry eye disease (DED) is a widespread and frequently reported multifactorial ocular disease that not only causes ocular discomfort but also damages the cornea and conjunctiva. Topical administration is the most common treatment modality for DED. Due to the existence of multiple biological barriers, instilled drugs generally exhibit short action times and poor penetration on the ocular surface.
- dry eye disease
- drug delivery
- new dosage forms
1. Introduction
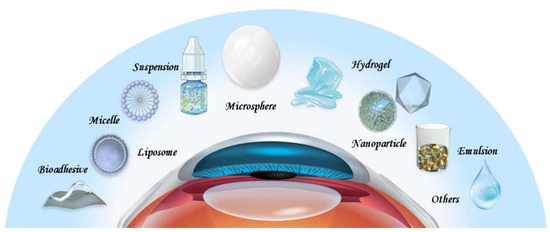
2. Drug Delivery Systems for DED
2. Drug Delivery Systems for DED
2.1. Suspensions
2.1. Suspensions
Suspension refers to a liquid formulation formed by dispersing insoluble drug particles in a liquid medium. Common methods for the preparation of suspension include direct dispersion, precipitation, and controlled flocculation. Drug particles in the suspension settle slowly at a rate that does not interfere with correct dosing. They do not agglomerate and can be dispersed evenly through shaking, even after long-term storage. After topical application, drug particles in the suspension can be retained in the cul-de-sac, which leads to prolonged ocular action duration
[28]
[32]
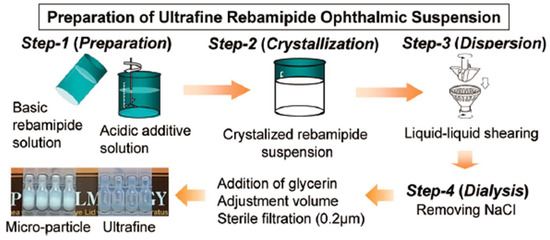
[33] formulated rebamipide particles into an ultrafine state (approximately 640 nm in size) to obtain a highly transparent (light transmittance: 59%) suspension. The duration of blurred vision was thus reduced. Moreover, an in vivo pharmacokinetics study revealed that the concentrations of rebamipide in cornea and conjunctiva were higher than those of conventional suspension, which indicated accelerated absorption rates and improved bioavailability. The particle size and transparency of this suspension remain unchanged for 3 years when stored at 25 °C, which demonstrated its excellent physicochemical stability. Augmenting the mucus-penetrating abilities of drugs represents another strategy to obtain improved outcomes. In this regard, Eysuvis® (Kala Pharmaceuticals, Arlington, MA, USA) is an original loteprednol nanosuspension developed by Kala using the AMPPLIFY mucus-penetrating particle drug delivery technology. This technology permits loteprednol to reach the ocular surface without being degraded in the tear film. A single application of Eysuvis® can increase the concentrations of loteprednol in the aqueous humor, cornea, and conjunctiva by up to three times compared with the commercial product Lotemax® (0.38% loteprednol etabonate eye gel, Bausch & Lomb, Clearwater, FL, USA). Eysuvis® showed high efficacy in DED treatment
[36]. However, the long-term in vivo safety of it remains to be determined.
2.2. Emulsions
[37]
[38]
[39]
[40]
[43]
2.3. Liposomes
[49]
[50]
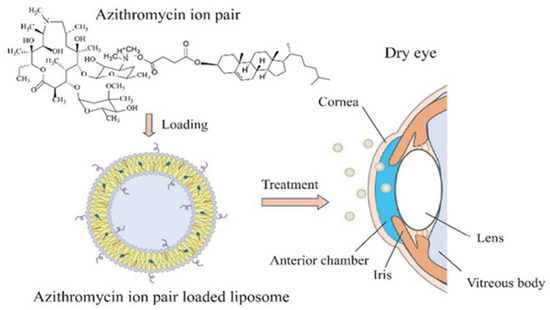
[56]. Ren T et al. formulated adriamycin ion pair loaded liposomes to improve the therapeutic effects of the drug against DED. First, an adriamycin–cholesterol hemisuccinate ion pair was prepared to improve the drug loading. Second, liposomes were prepared by a film hydration method. Finally, the liposomes were sonicated to obtain uniform particle size with high drug loading efficiency
[57]
2.4. Nanoparticles
[35]
[62].
Drug-loaded nanoparticles have also been formulated into dosage forms other than eye drops. For example, Ryu et al. developed nanoparticles incorporated tablets, by embedding PLGA nanoparticles containing dexamethasone in an alginate matrix. The table was applied to the ocular surface using a preocular applicator. It was found that the nanoparticles remained on the ocular surface for up to 2 h. This mode of administration not only improves the bioavailability of drugs but also enables their sterile delivery [63][64]. Nanocapsules are nanoparticles with hollow cores, which are mainly used to deliver labile drugs or engineered for targeted delivery. Although several nanocapsule-based delivery systems exist for anti-inflammatory drugs, few reports are available with respect to their application for the treatment of DED [65][66]. Zhang et al. prepared cyclosporine lipid nanocapsule eye drops with the phase-inversion method, which increased bioavailabilities of cyclosporine in the conjunctiva and cornea. In line with the pharmacokinetic study, superior therapeutic effects over conventional cyclosporine emulsion were observed in pharmacodynamic studies [67].
3. Conclusions
Other drug delivery systems, such as microspheres, hydrogels, and bioadhesive polymers have also been engineered to improve therapeutic effects of DED drugs. Indeed, promising results are obtained, which have the potential to lead to innovative therapies. Considering the shortcomings of each drug delivery system, the combination of two or more of them deserves further research. Current treatments for DED generally target one aspect of DED pathophysiology. It is fascinating to explore whether superior outcomes can be obtained with drug delivery systems that target multiple aspects simultaneously. There is also a lack of biodegradability and in vivo safety information concerning the above-mentioned drug delivery systems, as most studies are conducted in the short-term setting. A direct comparison between these delivery vehicles represents another issue to be addressed to determine the most suitable one. Finding answers to these questions constitutes the key areas of future research to improve drug delivery for DED.
References
- Hakim, F.; Farooq, A. Dry Eye Disease: An Update in 2022. JAMA 2022, 327, 478–479.
- Messmer, E. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81.
- Tsubota, K.; Pflugfelder, S.; Liu, Z.; Baudouin, C.; Kim, H.; Messmer, E.; Kruse, F.; Liang, L.; Carreno-Galeano, J.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271.
- Han, K.-T.; Nam, J.H.; Park, E.-C. Do Sleep Disorders Positively Correlate with Dry Eye Syndrome? Results of National Claim Data. Int. J. Environ. Res. Public Health 2019, 16, 878.
- Schaumberg, D.; Dana, R.; Buring, J.; Sullivan, D. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768.
- Lee, J.-H.; Lee, W.; Yoon, J.-H.; Seok, H.; Roh, J.; Won, J.-U. Relationship between symptoms of dry eye syndrome and occupational characteristics: The Korean National Health and Nutrition Examination Survey 2010–2012. BMC Ophthalmol. 2015, 15, 147.
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and Risk Factors of Dry Eye Disease in Japan: Koumi Study. Ophthalmology 2011, 118, 2361–2367.
- Maychuk, D.Y.; Anisimova, S.; Kapkova, S.; Kachanov, A.; Korotkikh, S.; Seleznev, A.; Sakhnov, S.; Leonova, E.; Krylov, S. Prevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in Russia. J. Cataract Refract. Surg. 2016, 42, 427–434.
- Wu, H.-X.; Xia, X.; Liu, K.; Zheng, Z.; Zhu, N.-Q.; Xu, X.; Gu, Q. Effect of insulin on VEGF expression in bovine retinal microvascular endothelial cells exposed to normal or high glucose. Chin. J. Ophthalmol. 2008, 44, 640–644.
- Li, J.; Zheng, K.; Deng, Z.; Zheng, J.; Ma, H.; Sun, L.; Chen, W. Prevalence and Risk Factors of Dry Eye Disease Among a Hospital-Based Population in Southeast China. Eye Contact Lens Sci. Clin. Pract. 2015, 41, 44–50.
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365.
- Wang, M.T.M.; Craig, J.P. Core Outcome Sets for Clinical Trials in Dry Eye Disease. JAMA Ophthalmol. 2018, 136, 1180–1181.
- O’Neil, E.C.; Henderson, M.; Massaro-Giordano, M.; Bunya, V.Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 2019, 30, 166–178.
- Thulasi, P.; Djalilian, A.R. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology 2017, 124, S27–S33.
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41.
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457.
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223.
- Nasir, N.A.A.; Agarwal, P.; Agarwal, R.; Iezhitsa, I.; Alyautdin, R.; Nukolova, N.N.; Chekhonin, V.P.; Ismail, N.M. Intraocular distribution of topically applied hydrophilic and lipophilic substances in rat eyes. Drug Deliv. 2016, 23, 2765–2771.
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423.
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Singh, T.R.R. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889.
- Nosch, D.S.; Joos, R.E.; Job, M. Prospective randomized study to evaluate the efficacy and tolerability of Ectoin® containing Eye Spray (EES09) and comparison to the liposomal Eye Spray Tears Again® (TA) in the treatment of dry eye disease. Contact Lens Anterior Eye 2021, 44, 101318.
- Pflugfelder, S.; de Paiva, C. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13.
- Şimşek, C.; Doğru, M.; Kojima, T.; Tsubota, K. Current Management and Treatment of Dry Eye Disease. Turk. J. Ophthalmol. 2018, 48, 309–313.
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior Eye Segment Drug Delivery Systems: Current Treatments and Future Challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105.
- Yellepeddi, V.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82.
- Baino, F.; Kargozar, S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering 2020, 7, 65.
- de Paiva, C.; Pflugfelder, S.; Ng, S.; Akpek, E. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst. Rev. 2019, 9, Cd010.
- Bron, A.; de Paiva, C.; Chauhan, S.; Bonini, S.; Gabison, E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510.Bron, A.; de Paiva, C.; Chauhan, S.; Bonini, S.; Gabison, E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510.
- Beckman, K.; Katz, J.; Majmudar, P.; Rostov, A. Loteprednol Etabonate for the Treatment of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2020, 36, 497–511.Beckman, K.; Katz, J.; Majmudar, P.; Rostov, A. Loteprednol Etabonate for the Treatment of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2020, 36, 497–511.
- Wan, P.-X.; Wang, X.-R.; Song, Y.-Y.; Li, Z.-Y.; Duan, H.-C.; Zhang, W.; Liu, Z.; Wang, Z.-C. Study on the treatment of dry eye with Loteprednol Etabonate. [Zhonghua Yan Ke Za Zhi] Chin. J. Ophthalmol. 2012, 48, 142–147.Wan, P.-X.; Wang, X.-R.; Song, Y.-Y.; Li, Z.-Y.; Duan, H.-C.; Zhang, W.; Liu, Z.; Wang, Z.-C. Study on the treatment of dry eye with Loteprednol Etabonate. Chin. J. Ophthalmol. 2012, 48, 142–147.
- Kashima, T.; Akiyama, H.; Kishi, S.; Itakura, H. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: A critical appraisal. Clin. Ophthalmol. 2014, 8, 1003–1010.Kashima, T.; Akiyama, H.; Kishi, S.; Itakura, H. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: A critical appraisal. Clin. Ophthalmol. 2014, 8, 1003–1010.
- Kinoshita, S.; Oshiden, K.; Awamura, S.; Suzuki, H.; Nakamichi, N.; Yokoi, N. A Randomized, Multicenter Phase 3 Study Comparing 2% Rebamipide (OPC-12759) with 0.1% Sodium Hyaluronate in the Treatment of Dry Eye. Ophthalmology 2013, 120, 1158–1165.Kinoshita, S.; Oshiden, K.; Awamura, S.; Suzuki, H.; Nakamichi, N.; Yokoi, N. A Randomized, Multicenter Phase 3 Study Comparing 2% Rebamipide (OPC-12759) with 0.1% Sodium Hyaluronate in the Treatment of Dry Eye. Ophthalmology 2013, 120, 1158–1165.
- Matsuda, T.; Hiraoka, S.; Urashima, H.; Ogura, A.; Ishida, T. Preparation of an Ultrafine Rebamipide Ophthalmic Suspension with High Transparency. Biol. Pharm. Bull. 2017, 40, 665–674.Matsuda, T.; Hiraoka, S.; Urashima, H.; Ogura, A.; Ishida, T. Preparation of an Ultrafine Rebamipide Ophthalmic Suspension with High Transparency. Biol. Pharm. Bull. 2017, 40, 665–674.
- Paton, D. Loteprednol etabonate: A formulation for short-term use in inflammatory flares in dry eye disease. Drugs Today 2022, 58, 77.Paton, D. Loteprednol etabonate: A formulation for short-term use in inflammatory flares in dry eye disease. Drugs Today 2022, 58, 77.
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538.Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538.
- Korenfeld, M.; Nichols, K.K.O.; Goldberg, D.; Evans, D.O.; Sall, K.; Foulks, G.; Coultas, S.; Brazzell, K. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients with Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea 2021, 40, 564–570.Korenfeld, M.; Nichols, K.K.O.; Goldberg, D.; Evans, D.O.; Sall, K.; Foulks, G.; Coultas, S.; Brazzell, K. Safety of KPI-121 Ophthalmic Suspension 0.25% in Patients with Dry Eye Disease: A Pooled Analysis of 4 Multicenter, Randomized, Vehicle-Controlled Studies. Cornea 2021, 40, 564–570.
- Yamaguchi, M.; Yasueda, S.-I.; Isowaki, A.; Yamamoto, M.; Kimura, M.; Inada, K.; Ohtori, A. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int. J. Pharm. 2005, 301, 121–128.Yamaguchi, M.; Yasueda, S.-I.; Isowaki, A.; Yamamoto, M.; Kimura, M.; Inada, K.; Ohtori, A. Formulation of an ophthalmic lipid emulsion containing an anti-inflammatory steroidal drug, difluprednate. Int. J. Pharm. 2005, 301, 121–128.
- Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318.Lallemand, F.; Felt-Baeyens, O.; Besseghir, K.; Behar-Cohen, F.; Gurny, R. Cyclosporine A delivery to the eye: A pharmaceutical challenge. Eur. J. Pharm. Biopharm. 2003, 56, 307–318.
- Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185.Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185.
- Baudouin, C.; De La Maza, M.S.; Amrane, M.; Garrigue, J.-S.; Ismail, D.; Figueiredo, F.C.; Leonardi, A. One-Year Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease. Eur. J. Ophthalmol. 2017, 27, 678–685.Baudouin, C.; De La Maza, M.S.; Amrane, M.; Garrigue, J.-S.; Ismail, D.; Figueiredo, F.C.; Leonardi, A. One-Year Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease. Eur. J. Ophthalmol. 2017, 27, 678–685.
- Leonardi, A.; Van Setten, G.; Amrane, M.; Ismail, D.; Garrigue, J.-S.; Figueiredo, F.C.; Baudouin, C. Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease: A Multicenter Randomized Trial. Eur. J. Ophthalmol. 2016, 26, 287–296.Leonardi, A.; Van Setten, G.; Amrane, M.; Ismail, D.; Garrigue, J.-S.; Figueiredo, F.C.; Baudouin, C. Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease: A Multicenter Randomized Trial. Eur. J. Ophthalmol. 2016, 26, 287–296.
- Hoy, S.M. Ciclosporin Ophthalmic Emulsion 0.1%: A Review in Severe Dry Eye Disease. Drugs 2017, 77, 1909–1916.Hoy, S.M. Ciclosporin Ophthalmic Emulsion 0.1%: A Review in Severe Dry Eye Disease. Drugs 2017, 77, 1909–1916.
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805.Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.-Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805.
- Nazlı, H.; Mesut, B.; Özsoy, Y. In Vitro Evaluation of a Solid Supersaturated Self Nanoemulsifying Drug Delivery System (Super-SNEDDS) of Aprepitant for Enhanced Solubility. Pharmaceuticals 2021, 14, 1089.Nazlı, H.; Mesut, B.; Özsoy, Y. In Vitro Evaluation of a Solid Supersaturated Self Nanoemulsifying Drug Delivery System (Super-SNEDDS) of Aprepitant for Enhanced Solubility. Pharmaceuticals 2021, 14, 1089.
- Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703.Rasoanirina, B.N.V.; Lassoued, M.A.; Kamoun, A.; Bahloul, B.; Miladi, K.; Sfar, S. Voriconazole-loaded self-nanoemulsifying drug delivery system (SNEDDS) to improve transcorneal permeability. Pharm. Dev. Technol. 2020, 25, 694–703.
- Arshad, R.; Tabish, T.; Kiani, M.; Ibrahim, I.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086.Arshad, R.; Tabish, T.; Kiani, M.; Ibrahim, I.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086.
- Amrane, M.; Creuzot-Garcher, C.; Robert, P.-Y.; Ismail, D.; Garrigue, J.-S.; Pisella, P.-J.; Baudouin, C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease—A randomised comparative study. J. Fr. Ophtalmol. 2014, 37, 589–598.Amrane, M.; Creuzot-Garcher, C.; Robert, P.-Y.; Ismail, D.; Garrigue, J.-S.; Pisella, P.-J.; Baudouin, C. Ocular tolerability and efficacy of a cationic emulsion in patients with mild to moderate dry eye disease—A randomised comparative study. J. Fr. Ophtalmol. 2014, 37, 589–598.
- Lyseng-Williamson, K.A. Cationorm® (cationic emulsion eye drops) in dry eye disease: A guide to its use. Drugs Ther. Perspect. 2016, 32, 317–322.Lyseng-Williamson, K.A. Cationorm® (cationic emulsion eye drops) in dry eye disease: A guide to its use. Drugs Ther. Perspect. 2016, 32, 317–322.
- Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W.M. General Perception of Liposomes: Formation, Manufacturing and Applications. In Liposomes-Advances and Perspectives; IntechOpen: Rijeka, Croatia, 2019.Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W.M. General Perception of Liposomes: Formation, Manufacturing and Applications. In Liposomes-Advances and Perspectives; IntechOpen: Rijeka, Croatia, 2019.
- Liang, W.; Levchenko, T.S.; Torchilin, V.P. Encapsulation of ATP into liposomes by different methods: Optimization of the procedure. J. Microencapsul. 2004, 21, 251–261.Liang, W.; Levchenko, T.S.; Torchilin, V.P. Encapsulation of ATP into liposomes by different methods: Optimization of the procedure. J. Microencapsul. 2004, 21, 251–261.
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571.
- López-Cano, J.; González-Cela-Casamayor, M.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847.López-Cano, J.; González-Cela-Casamayor, M.; Andrés-Guerrero, V.; Herrero-Vanrell, R.; Molina-Martínez, I. Liposomes as vehicles for topical ophthalmic drug delivery and ocular surface protection. Expert Opin. Drug Deliv. 2021, 18, 819–847.
- Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Nasir, N.A.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091.Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Nasir, N.A.A.; Razali, N.; Alyautdin, R.; Ismail, N.M. Liposomes in topical ophthalmic drug delivery: An update. Drug Deliv. 2016, 23, 1075–1091.
- Craig, J.P.; Purslow, C.; Murphy, P.J.; Wolffsohn, J.S. Effect of a liposomal spray on the pre-ocular tear film. Contact Lens Anterior Eye 2010, 33, 83–87.Craig, J.P.; Purslow, C.; Murphy, P.J.; Wolffsohn, J.S. Effect of a liposomal spray on the pre-ocular tear film. Contact Lens Anterior Eye 2010, 33, 83–87.
- Khaireddin, R.; Schmidt, K. Comparative investigation of treatments for evaporative dry eye. Klin. Mon. Augenheilkd. 2010, 227, 128–134.Khaireddin, R.; Schmidt, K. Comparative investigation of treatments for evaporative dry eye. Klin. Mon. Augenheilkd. 2010, 227, 128–134.
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168.Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168.
- Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol. Pharm. 2018, 15, 4862–4871.Ren, T.; Lin, X.; Zhang, Q.; You, D.; Liu, X.; Tao, X.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Encapsulation of Azithromycin Ion Pair in Liposome for Enhancing Ocular Delivery and Therapeutic Efficacy on Dry Eye. Mol. Pharm. 2018, 15, 4862–4871.
- López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698.López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698.
- Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye disease model. J. Clin. Biochem. Nutr. 2020, 66, 224–232.Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye disease model. J. Clin. Biochem. Nutr. 2020, 66, 224–232.
- Shimokawa, T.; Yoshida, M.; Fukuta, T.; Tanaka, T.; Inagi, T.; Kogure, K. Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. J. Clin. Biochem. Nutr. 2019, 64, 27–35.Shimokawa, T.; Yoshida, M.; Fukuta, T.; Tanaka, T.; Inagi, T.; Kogure, K. Efficacy of high-affinity liposomal astaxanthin on up-regulation of age-related markers induced by oxidative stress in human corneal epithelial cells. J. Clin. Biochem. Nutr. 2019, 64, 27–35.
- Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155.Nagai, N.; Ishii, M.; Seiriki, R.; Ogata, F.; Otake, H.; Nakazawa, Y.; Okamoto, N.; Kanai, K.; Kawasaki, N. Novel Sustained-Release Drug Delivery System for Dry Eye Therapy by Rebamipide Nanoparticles. Pharmaceutics 2020, 12, 155.
- Li, Y.-J.; Luo, L.-J.; Harroun, S.G.; Wei, S.-C.; Unnikrishnan, B.; Chang, H.-T.; Huang, Y.-F.; Lai, J.-Y.; Huang, C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale 2019, 11, 5580–5594.Li, Y.-J.; Luo, L.-J.; Harroun, S.G.; Wei, S.-C.; Unnikrishnan, B.; Chang, H.-T.; Huang, Y.-F.; Lai, J.-Y.; Huang, C.-C. Synergistically dual-functional nano eye-drops for simultaneous anti-inflammatory and anti-oxidative treatment of dry eye disease. Nanoscale 2019, 11, 5580–5594.
- Ryu, W.M.; Kim, S.-N.; Min, C.H.; Bin Choy, Y. Dry Tablet Formulation of PLGA Nanoparticles with a Preocular Applicator for Topical Drug Delivery to the Eye. Pharmaceutics 2019, 11, 651.
- Coursey, T.G.; Henriksson, J.T.; Marcano, D.C.; Shin, C.S.; Isenhart, L.C.; Ahmed, F.; De Paiva, C.S.; Pflugfelder, S.C.; Acharya, G. Dexamethasone nanowafer as an effective therapy for dry eye disease. J. Control Release 2015, 213, 168–174.
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical tacrolimus nanocapsules eye drops for therapeutic effect enhancement in both anterior and posterior ocular inflammation models. J. Control Release 2021, 333, 283–297.
- Hu, Q.; Wu, W.; Wang, M.; Shao, S.; Jin, P.; Chen, Q.; Bai, H.; Zhao, X.; Huang, J.; Wang, J.; et al. Reverting chemoresistance of targeted agents by a ultrasoluble dendritic nanocapsule. J. Control Release 2020, 317, 67–77.
- Zhang, R.; Sun, M.; Ran, Y.; Deng, Y.; Ge, Y.; Zhu, X.; Tao, L.; Shang, J.; Gou, H.; He, T.; et al. A Novel Eyes Topical Drug Delivery System: CsA-LNC for the Treatment of DED. Pharm. Res. 2020, 37, 146.
