Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Dry eye disease (DED) is a widespread and frequently reported multifactorial ocular disease that not only causes ocular discomfort but also damages the cornea and conjunctiva. Topical administration is the most common treatment modality for DED. Due to the existence of multiple biological barriers, instilled drugs generally exhibit short action times and poor penetration on the ocular surface. To resolve these issues, several advanced drug delivery systems have been proposed.
- dry eye disease
- drug delivery
- new dosage forms
Dry eye disease (DED) is resulted from decreased tear production, excessive evaporation of tears, or both, ultimately leading to inflammation of the ocular surface [1]. It is manifested by diverse and complex pathological processes. Presently, the association of these processes is not fully established. In 1995, the American Academy of Ophthalmology (AAO) classified DED into two subtypes, namely evaporative dry eye and aqueous-deficient dry eye. However, most patients with DED present symptoms of both subtypes [2]. Epithelial lesions of the ocular surface, inflammation, and neurosensory abnormalities caused by DED can lead to the manifestation of diverse clinical symptoms, such as redness, pain, blurred vision, and sleep disorders. These discomforts not only reduce the patients’ productivity but also seriously affect their quality of life [3][4]. Epidemiological studies reveal that the prevalence of DED is approximately 7% in Europe and America, 12.5–21.6% in Japan and Republic of Korea, and 21–30% in China. In some areas of Russia, the prevalence has been reported to be 40–55% [5][6][7][8][9]. Aging, female gender, prior eye surgery, and widespread use of video terminals have been reported as high-risk factors contributing to the development of DED [10]. China has a huge and aging population, where the prevention and treatment of DED are discouraging. With the significant progress in epidemiological studies of DED in recent years, it is recognized that DED has become an important public health concern [11][12].
Presently, various treatment modalities, such as warm compresses, meibomian gland expression, intranasal tear neurostimulation, contact lenses, and topical medications have been prescribed for DED, among which the topical application of medications represents the most important one [13][14][15][16]. However, multiple biological barriers on the ocular surface, such as the tear film barrier and the corneal and conjunctival barrier, result in rapid drug clearance and low bioavailability. Specifically, the presence of the tear film leads to rapid drug loss and the dense epithelium of the conjunctiva hinders drug entry [17]. Cornea is in fact an amphiphilic tissue, the epithelium layer of which is hydrophobic, so it is difficult for hydrophilic drugs to stay or diffuse. However, if the drug has high lipophilicity, it is difficult for it to penetrate the hydrophilic stromal layer. As a result, drugs must have balanced hydrophilicity and lipophilicity to pass through the entire cornea [18]. These determine that topically applied drugs generally demonstrate extremely low bioavailability. To improve the bioavailability of drugs in the eye, numerous drug delivery systems have been developed (Figure 1) [19][20][21]. For example, nanoparticles are employed to improve the corneal penetration of drugs, hydrogels are employed to extend the retention time of drugs on the ocular surface, and microspheres are employed to achieve sustained release. While eye drops remain the most common delivery vehicles for DED drugs, drug-loaded implants, drug sprays, and microneedles are increasingly being explored. DED is characterized by a vicious cycle triggered by the multifactorial disruption of the ocular surface microenvironment, which leads to inflammation and decreased tear film stability [22]. To ameliorate this chronic condition, long-term treatments are inevitable. However, the long-term use of medications can be associated with serious side effects. The DEWS II report suggests that short-term and long-term treatments should be combined flexibly for different patient conditions to maximize the therapeutic benefits [23]. For patients with mild DED, the use of artificial tears alone can achieve satisfactory therapeutic effects. In contrast, for patients with moderate-to-severe DED, single or multiple drugs are often warranted. As a result, there is an attempt to improve the delivery of drugs with rationally designed drug delivery systems according to the properties of intended drugs and the disease conditions for better outcomes. Some of these delivery systems have led to innovative therapies which are under clinical investigation or even enter the clinic [21][24][25][26][27].
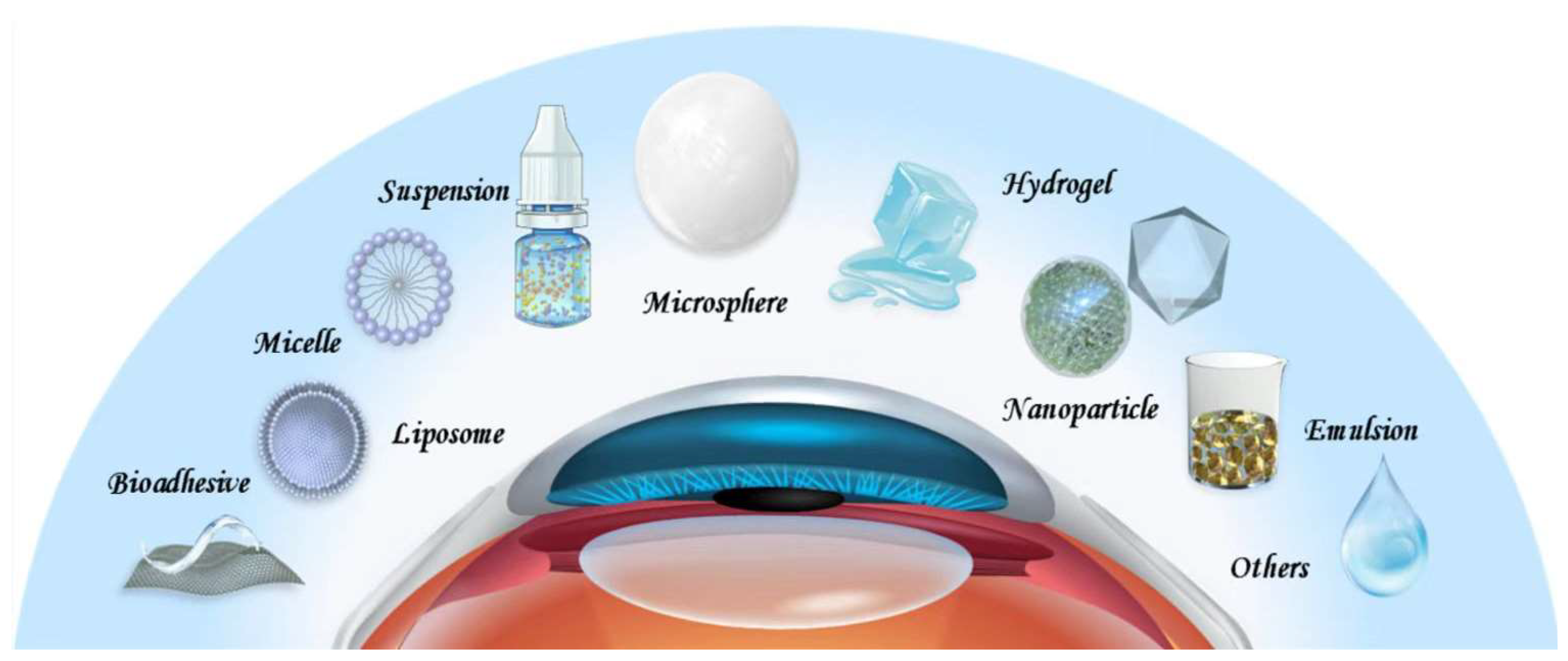
Figure 1. Drug delivery systems for DED.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering10010053
References
- Hakim, F.; Farooq, A. Dry Eye Disease: An Update in 2022. JAMA 2022, 327, 478–479.
- Messmer, E. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81.
- Tsubota, K.; Pflugfelder, S.; Liu, Z.; Baudouin, C.; Kim, H.; Messmer, E.; Kruse, F.; Liang, L.; Carreno-Galeano, J.; Rolando, M.; et al. Defining Dry Eye from a Clinical Perspective. Int. J. Mol. Sci. 2020, 21, 9271.
- Han, K.-T.; Nam, J.H.; Park, E.-C. Do Sleep Disorders Positively Correlate with Dry Eye Syndrome? Results of National Claim Data. Int. J. Environ. Res. Public Health 2019, 16, 878.
- Schaumberg, D.; Dana, R.; Buring, J.; Sullivan, D. Prevalence of dry eye disease among US men: Estimates from the Physicians’ Health Studies. Arch. Ophthalmol. 2009, 127, 763–768.
- Lee, J.-H.; Lee, W.; Yoon, J.-H.; Seok, H.; Roh, J.; Won, J.-U. Relationship between symptoms of dry eye syndrome and occupational characteristics: The Korean National Health and Nutrition Examination Survey 2010–2012. BMC Ophthalmol. 2015, 15, 147.
- Uchino, M.; Nishiwaki, Y.; Michikawa, T.; Shirakawa, K.; Kuwahara, E.; Yamada, M.; Dogru, M.; Schaumberg, D.A.; Kawakita, T.; Takebayashi, T.; et al. Prevalence and Risk Factors of Dry Eye Disease in Japan: Koumi Study. Ophthalmology 2011, 118, 2361–2367.
- Maychuk, D.Y.; Anisimova, S.; Kapkova, S.; Kachanov, A.; Korotkikh, S.; Seleznev, A.; Sakhnov, S.; Leonova, E.; Krylov, S. Prevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in Russia. J. Cataract Refract. Surg. 2016, 42, 427–434.
- Wu, H.-X.; Xia, X.; Liu, K.; Zheng, Z.; Zhu, N.-Q.; Xu, X.; Gu, Q. Effect of insulin on VEGF expression in bovine retinal microvascular endothelial cells exposed to normal or high glucose. Chin. J. Ophthalmol. 2008, 44, 640–644.
- Li, J.; Zheng, K.; Deng, Z.; Zheng, J.; Ma, H.; Sun, L.; Chen, W. Prevalence and Risk Factors of Dry Eye Disease Among a Hospital-Based Population in Southeast China. Eye Contact Lens Sci. Clin. Pract. 2015, 41, 44–50.
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II epidemiology report. Ocul. Surf. 2017, 15, 334–365.
- Wang, M.T.M.; Craig, J.P. Core Outcome Sets for Clinical Trials in Dry Eye Disease. JAMA Ophthalmol. 2018, 136, 1180–1181.
- O’Neil, E.C.; Henderson, M.; Massaro-Giordano, M.; Bunya, V.Y. Advances in dry eye disease treatment. Curr. Opin. Ophthalmol. 2019, 30, 166–178.
- Thulasi, P.; Djalilian, A.R. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology 2017, 124, S27–S33.
- Dosmar, E.; Walsh, J.; Doyel, M.; Bussett, K.; Oladipupo, A.; Amer, S.; Goebel, K. Targeting Ocular Drug Delivery: An Examination of Local Anatomy and Current Approaches. Bioengineering 2022, 9, 41.
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design principles of ocular drug delivery systems: Importance of drug payload, release rate, and material properties. Drug Discov. Today 2019, 24, 1446–1457.
- Awwad, S.; Ahmed, A.H.A.M.; Sharma, G.; Heng, J.S.; Khaw, P.T.; Brocchini, S.; Lockwood, A. Principles of pharmacology in the eye. Br. J. Pharmacol. 2017, 174, 4205–4223.
- Nasir, N.A.A.; Agarwal, P.; Agarwal, R.; Iezhitsa, I.; Alyautdin, R.; Nukolova, N.N.; Chekhonin, V.P.; Ismail, N.M. Intraocular distribution of topically applied hydrophilic and lipophilic substances in rat eyes. Drug Deliv. 2016, 23, 2765–2771.
- Holland, E.J.; Darvish, M.; Nichols, K.K.; Jones, L.; Karpecki, P.M. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: A systematic literature review. Ocul. Surf. 2019, 17, 412–423.
- Yellepeddi, V.K.; Sheshala, R.; McMillan, H.; Gujral, C.; Jones, D.; Singh, T.R.R. Punctal plug: A medical device to treat dry eye syndrome and for sustained drug delivery to the eye. Drug Discov. Today 2015, 20, 884–889.
- Nosch, D.S.; Joos, R.E.; Job, M. Prospective randomized study to evaluate the efficacy and tolerability of Ectoin® containing Eye Spray (EES09) and comparison to the liposomal Eye Spray Tears Again® (TA) in the treatment of dry eye disease. Contact Lens Anterior Eye 2021, 44, 101318.
- Pflugfelder, S.; de Paiva, C. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13.
- Şimşek, C.; Doğru, M.; Kojima, T.; Tsubota, K. Current Management and Treatment of Dry Eye Disease. Turk. J. Ophthalmol. 2018, 48, 309–313.
- Molokhia, S.A.; Thomas, S.C.; Garff, K.J.; Mandell, K.J.; Wirostko, B.M. Anterior Eye Segment Drug Delivery Systems: Current Treatments and Future Challenges. J. Ocul. Pharmacol. Ther. 2013, 29, 92–105.
- Yellepeddi, V.; Palakurthi, S. Recent Advances in Topical Ocular Drug Delivery. J. Ocul. Pharmacol. Ther. 2016, 32, 67–82.
- Baino, F.; Kargozar, S. Regulation of the Ocular Cell/Tissue Response by Implantable Biomaterials and Drug Delivery Systems. Bioengineering 2020, 7, 65.
- de Paiva, C.; Pflugfelder, S.; Ng, S.; Akpek, E. Topical cyclosporine A therapy for dry eye syndrome. Cochrane Database Syst. Rev. 2019, 9, Cd010.
This entry is offline, you can click here to edit this entry!
