
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ugo Picciotti | -- | 2081 | 2023-02-14 10:56:29 | | | |
| 2 | Ugo Picciotti | Meta information modification | 2081 | 2023-02-14 11:06:29 | | | | |
| 3 | Camila Xu | Meta information modification | 2081 | 2023-02-15 01:25:48 | | | | |
| 4 | Camila Xu | Meta information modification | 2081 | 2023-02-15 01:44:58 | | | | |
| 5 | Camila Xu | Meta information modification | 2081 | 2023-02-16 07:15:36 | | | | |
| 6 | Ugo Picciotti | + 197 word(s) | 2278 | 2023-02-21 12:00:31 | | |
Video Upload Options
Multitrophic interactions link several trophic levels, including plants, phytophagous, predators, parasitoids, and/or pathogens.
1. Introduction
Trophic levels represent a hierarchical stratification through which organisms are positioned in the food chain. Producers (plants) are the basal resource, herbivores feed on plants and form a secondary trophic level (primary consumers), carnivores eat herbivores, forming a third trophic level (secondary consumers), and additional increasing apex levels defined by how many consumers we find in that given community.
Multitrophic interactions link multiple trophic levels, describing how the exchange of matter and energy occurs. They include simple tri-trophic relationships (producers, herbivores, consumers) but extend to more levels, such as higher-order predators preying on intermediate predators and hyperparasitoids attacking primary parasitoids.
Omnivory (the consumption of plants and animals) is a widespread feeding strategy, represented in 12 insect orders and at least 40 families [5]. This behavior increases the complexity of possible trophic links for each species. Price et al. [6] remind us that insects complicate the placement of species into individual functional groups because of their ontogenetic development. Several insects, changing from juvenile to adult, shift food habits placing themselves in different food guilds depending on their stage of development.
Microbes greatly affect the terrestrial ecosystem at the multitrophic level; they link plants to other trophic levels, e.g., heterotrophs degrade the organic matter waste from the higher trophic levels to the producers, making it possible for plants to return inorganic nutrients (degraders trophic level).
Microorganisms can also establish relationships among animals or plants that become their hosts.2. Interactions between Entomopathogenic Fungi and Pests
3. Multitrophic Interactions of Entomopathogenic Fungi, Crops, and Insects
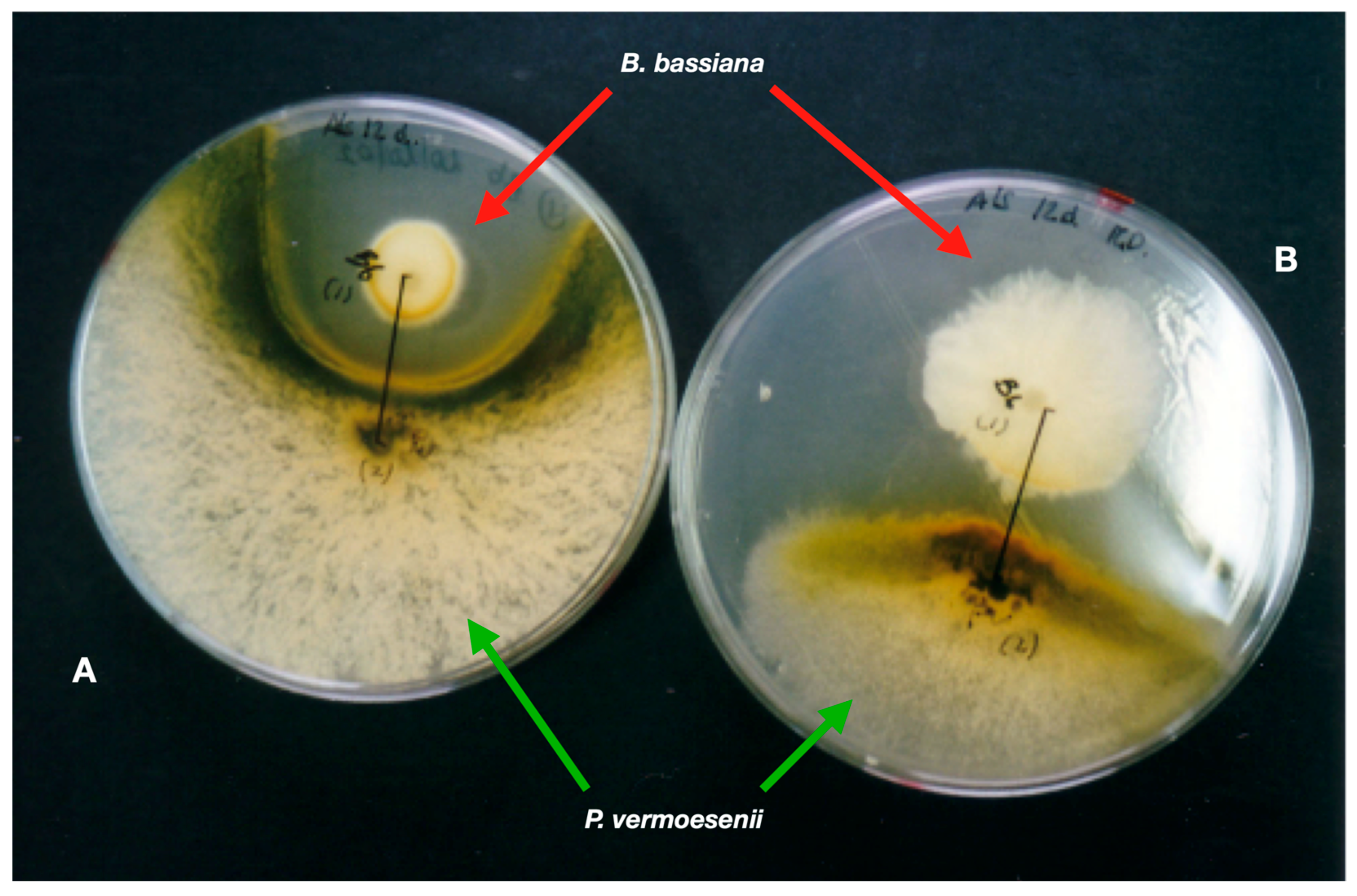
References
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al.et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484, 10.1126/science.1226727.
- Engel, M.S. Insect evolution. Current Biology 2015, 25, R868–R872, 10.1016/j.cub.2015.07.059 .
- Berenbaum, M.. Insect biodiversity—Millions and millions; Foottit, R.G.; Adler, P.H., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 783–792.
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The evolution of insect biodiversity. Current Biology 2021, 31, R1299–R1311, 10.1016/j.cub.2021.08.057 .
- Coll, M.; Guershon, M. Omnivory in Terrestrial Arthropods: Mixing Plant and Prey Diets. Annual Review of Entomology 2002, 47, 267-297, 10.1146/annurev.ento.47.091201.145209.
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. . Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: Cambridge, UK, 2011; pp. 307.
- de Bary, H.A.. Die Erscheinung der Symbiose: Vortrag, Gehalten auf der Versammlung Deutscher Naturforscher und Aerzte zu Cassel; Verlag von Karl J. Trübner: Strassburg, Germany, 1879; pp. 1–30.
- Tellez, G. Prokaryotes Versus Eukaryotes: Who is Hosting Whom?. Frontiers in Veterinary Science 2014, 1, 3, 10.3389/fvets.2014.00003.
- Hosokawa, T.; Fukatsu, T. Relevance of microbial symbiosis to insect behavior. Current Opinion in Insect Science 2020, 39, 91–100, 10.1016/j.cois.2020.03.004 .
- Douglas, A.E. Mycetocyte symbiosis in insects. Biological Reviews 1989, 64, 409–434, 10.1111/j.1469-185x.1989.tb00682.x .
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nature Reviews Genetics 2008, 9, 218–229, 10.1038/nrg2319.
- Engel, P.; Moran, N.A. The gut microbiota of insects - diversity in structure and function. FEMS Microbiology Ecology 2013, 37, 699–735, 10.1111/1574-6976.12025 .
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philosophical Transactions of the Royal Society B: Biological Sciences 2011, 366, 1389–1400, 10.1098/rstb.2010.0226 .
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends in Ecology & Evolution 2012, 27, 705–711, 10.1016/j.tree.2012.08.013 .
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie et Milieu/Life & Environment 2008, 58, 107-115.
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. BioControl 2010, 55, 89–102, 10.1007/s10526-009-9238-5.
- Roy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annual Review of Entomology 2006, 51, 331–357, 10.1146/annurev.ento.51.110104.150941.
- St. Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied Microbiology and Biotechnology 2010, 85, 901–907, 10.1007/s00253-009-2306-z.
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biological Control 2010, 55, 34–41, 10.1016/j.biocontrol.2010.06.011.
- Posada-Flórez, F.J. Production of Beauveria bassiana fungal spores on rice to control the coffee berry borer, Hypothenemus hampei, in Colombia. Journal of Insect Science 2008, 8, 41, 10.1673/031.008.4101.
- Yasuda, K. Auto-infection system for the sweet potato weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) with entomopathogenic fungi, Beauveria bassiana using a modified sex pheromone trap in the field. Applied Entomology and Zoology 1999, 34, 501–505.
- Kumar, V.; Singh, G.P.; Babu, A.M.; Ahsan, M.M.; Datta, R.K. Germination, penetration, and invasion of Beauveria bassiana on silkworm, Bombyx mori, causing white muscardine. Italian Journal of Zoology 1999, 66, 39–43, 10.1080/11250009909356235.
- Godonou, I.; Green, K.R.; Oduro, K.A.; Lomer, C.J.; Afreh-Nuamah, K. Field evaluation of selected formulations of Beauveria bassiana for the management of the banana weevil (Cosmopolites sordidus) on plantain (Musa spp., AAB group). Biocontrol Science and Technology 2000, 10, 779–788, 10.1080/09583150020011726.
- Tinzaara, W.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. Biocontrol Science and Technology 2007, 17, 111–124, 10.1080/09583150600937089.
- Fancelli, M.; Batista Dias, A.; Delalibera Júnior, I.; Cerqueira de Jesus, S.; Souza do Nascimento, A.; de Oliveira e Silva, S.; Correa Caldas, R.; da Silva Ledo, C.A. Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in plantain. BioMed Research International 2013, 2013, 184756, 10.1155/2013/184756 .
- Mc Namara, L.; Dolan, S.K.; Walsh, J.M.D.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Fungal Biology 2019, 123, 601–610, 10.1016/j.funbio.2019.01.004.
- Membang, G.; Ambang, Z.; Mahot, H.C.; Kuate, A.F.; Fiaboe, K.K.M.; Hanna, R. Cosmopolites sordidus (Germar) susceptibility to indigenous Cameroonian Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) isolates. Journal of Applied Entomology 2020, 144, 468–480, 10.1111/jen.12757.
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. Journal of Applied Entomology 2017, 141, 80–87, 10.1111/jen.12365.
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, e0190454, 10.1371/journal.pone.0190454.
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long chain alcohols produced by Trichoderma citrinoviride have phagodeterrent activity against the bird cherry-oat aphid Rhopalosiphum padi. Frontiers in Microbiology 2016, 7, 297, 10.3389/fmicb.2016.00297 .
- Evidente, A.; Andolfi, A.; Cimmino, A.; Ganassi, S.; Altomare, C.; Favilla, M.; De Cristofaro, A.; Vitagliano, S.; Sabatini, A.M. Bisorbicillinoids produced by the fungus Trichoderma citrinoviride affect feeding preference of the aphid Schizaphis graminum. Journal of Chemical Ecology 2009, 35, 533–541, 10.1007/s10886-009-9632-6.
- Asensio, L.; Carbonell, T.; López-Jiménez, J.A.; Lopez-Llorca, L.V. Entomopathogenic fungi in soils from Alicante province [Spain]. Spanish Journal of Agricultural Research 2003, 1, 9, 10.5424/sjar/2003013-33.
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509, 10.3390/insects11080509.
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Science and Technology 2007, 17, 553–596, 10.1080/09583150701309006.
- Lopez-Llorca, L.V. Thrips affected by entomopathogenic fungus Beauveria bassiana. Phytoma España 1993, 53, 41–43.
- Lopez-Llorca, L.V. Aphid infection by the entomopathogen Erynia neoaphidis—SEM study. Mycologist 1993, 7, 166–168, 10.1016/S0269-915X(09)80387-X.
- Lopez-Llorca, L.V.; Carbonell, T.; Gomez-Vidal, S. Degradation of insect cuticle by Paecilomyces farinosus proteases. Mycological Progress 2002, 1, 249–256, 10.1007/s11557-006-0022-y.
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. Microscopy Research and Technique 2010, 73, 714–725, 10.1002/jemt.20812.
- Ricaño, J.; Güerri-Agulló, B.; Serna-Sarriás, M.J.; Rubio-Llorca, G.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Florida Entomologist 2013, 96, 1311–1324, 10.1653/024.096.0410.
- Güerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Florida Entomologist 2011, 94, 737–747, 10.1653/024.094.0402.
- Aguilera Sammaritano, J.A.; López Lastra, C.C.; Leclerque, A.; Vazquez, F.; Toro, M.E.; D’Alessandro, C.P.; Cuthbertson, A.G.S.; Lechner, B.E. Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. Biocontrol Science and Technology 2016, 26, 1668–1682, 10.1080/09583157.2016.1231776.
- Casique-Valdés, R.; Torres-Acosta, R.I.; Sánchez-Peña, S.R. Metarhizium acridum and other entomopathogenic fungi from grasshop- pers at arid sites of northeastern Mexico. Southwestern Entomologist 2022, 47, 547–558, 10.3958/059.047.0302.
- Lopez-Llorca, L.V.; Carbonell, T.; Salinas, J. Colonization of plant waste substrates by entomopathogenic and mycoparasitic fungi—A SEM study. Micron 1999, 30, 325–333, 10.1016/S0968-4328(99)00031-1.
- Asensio, L.; López-Jiménez, J.Á.; López-Llorca, L.V. Mycobiota of the date palm phylloplane: description and interactions. Revista Iberoamericana de Micología 2007, 24, 299–304, 10.1016/s1130-1406(07)70060-8 .
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 2006, 37, 624–632, 10.1016/j.micron.2006.02.003.
- Mohamed Mahmoud, F.; Krimi, Z.; Maciá-Vicente, J.G.; Brahim Errahmani, M.; Lopez-Llorca, L.V. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Revista Iberoamericana de Micología 2017, 34, 116–120, 10.1016/j.riam.2016.06.007.
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. Journal of Applied Microbiology 2021, 130, 570–581, 10.1111/jam.14503.
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nature Reviews Microbiology 2013, 11, 789–799, 10.1038/nrmicro3109.
- Aranda-Martinez,A.; NaranjoOrtiz,M.Á.; AbihssiraGarcía,I.S.; Zavala-Gonzalez,E.A.; Lopez-Llorca,L.V. Ethanol production from chitosan by the nematophagous fungus Pochonia chlamydosporia and the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Microbiological Research 2017, 204, 30–39, 10.1016/j.micres.2017.07.009.
- González-Mas,N.; Gutiérrez-Sánchez,F.; Sánchez-Ortiz,A.; Grandi,L.; Turlings,T.C.J.; ManuelMuñoz-Redondo,J.; Moreno- Rojas, J.M.; Quesada-Moraga, E. Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. Frontiers in Plant Science 2021, 12, 660460, 10.3389/fpls.2021.660460.
- Rivas-Franco,F.; Hampton,J.G.; Altier,N.A.; Swaminathan,J.; Rostás,M.; Wessman,P.; Saville,D.J.; Jackson,T.A.; Jackson,M.A.; Glare, T.R.; et al. Production of microsclerotia from entomopathogenic fungi and use in maize seed coating as delivery for biocontrol against Fusarium graminearum. Frontiers in Sustainable Food Systems 2020, 4, 606828, 10.3389/fsufs.2020.606828.
- Mathulwe, L.L.; Malan, A.P.; Stokwe, N.F. Mass production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against insect pests. Journal of Visualized Experiments 2022, 181, e63246, 10.3791/63246.
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 9, 2996–3005, 10.1002/elps.200900192.
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycology 2018, 9, 189–201, 10.1080/21501203.2018.1473299.
- Palma-Guerrero, J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N.D. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genetics and Biology 2009, 46, 585–594, 10.1016/j.fgb.2009.02.010.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. International Journal of Molecular Sciences 2019, 20, 332, 10.3390/ijms20020332.
- Lopez-Nuñez, R.; Suarez-Fernandez, M.; Lopez-Moya, F.; Lopez-Llorca, L.V. Chitosan and nematophagous fungi for sustainable management of nematode pests. Frontiers in Fungal Biology 2022, 3, 980341, 10.3389/ffunb.2022.980341.
- Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan: Role of fungal cell wall on sensitivity to chitosan. Journal of Basic Microbiology 2016, 56, 1059–1070, 10.1002/jobm.201500775.
- Lopez-Moya, F.; Lopez-Llorca, L.V. Omics for investigating chitosan as an antifungal and gene modulator. Journal of Fungi 2016, 2, 11, 10.3390/jof2010011.
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biology 2016, 120, 572–585, 10.1016/j.funbio.2015.12.005.
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Frontiers in Plant Science 2017, 8, 1415, 10.3389/fpls.2017.01415.
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environmental Microbiology 2021, 23, 4980–4997, 10.1111/1462-2920.15408.
- Lin, R.; Qin, F.; Shen, B.; Shi, Q.; Liu, C.; Zhang, X.; Jiao, Y.; Lu, J.; Gao, Y.; Suarez-Fernandez, M.; et al. Genome and secretome analysis of Pochonia chlamydosporia provide new insight into egg-parasitic mechanisms. Scientific Reports 2018, 8, 1123, 10.1038/s41598-018-19169-5.
- Jalinas, J.; Guerri-Agullo, B.; Mankin, R.W.; Lopez-Follana, R.; Lopez-Llorca, L.V. Acoustic assessment of Beauveria Bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. Journal of Economic Entomology 2015, 108, 444–453, 10.1093/jee/tov023.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.




