MThe influltitrophicence between microorganisms and insects has led to the establishment of different types of interaction that can be summarised in various forms of interactions link. De Bary first defined symbiosis as “the cohabitation of distinct organisms”. Symbiosis isever here intended as a significant biological liaison between orophic levels, including plants, phytophagous, predators, parasitoids, and/or pathogens among species. Symbiosis is one of the leading evolutionary drivers promoting natural biological novelty. Symbiotic relationships between prokaryotes and eukaryotes are present in all kingdoms of life.
- alien
- invasive or quarantine pest
- Integrated Farming
- resilience
1. Introduction
Trophic levels represent a hierarchical stratification through which organisms are positioned in the food chain. Producers (plants) are the basal resource, herbivores feed on plants and form a secondary trophic level (primary consumers), carnivores eat herbivores, forming a third trophic level (secondary consumers), and additional increasing apex levels defined by how many consumers we find in that given community.
Multitrophic interactions link multiple trophic levels, describing how the exchange of matter and energy occurs. They include simple tri-trophic relationships (producers, herbivores, consumers) but extend to more levels, such as higher-order predators preying on intermediate predators and hyperparasitoids attacking primary parasitoids.
Omnivory (the consumption of plants and animals) is a widespread feeding strategy, represented in 12 insect orders and at least 40 families [5]. This behavior increases the complexity of possible trophic links for each species. Price et al. [6] remind us that insects complicate the placement of species into individual functional groups because of their ontogenetic development. Several insects, changing from juvenile to adult, shift food habits placing themselves in different food guilds depending on their stage of development.
Microbes greatly affect the terrestrial ecosystem at the multitrophic level; they link plants to other trophic levels, e.g., heterotrophs degrade the organic matter waste from the higher trophic levels to the producers, making it possible for plants to return inorganic nutrients (degraders trophic level).
Microorganisms can also establish relationships among animals or plants that become their hosts.2. Interactions between Entomopathogenic Fungi and Pests
Biological control of invasive pests also relies on certain entomopathogenic fungi (EFs) that can infect hosts in agroecosystems and appear suitable for plant protection exploitation. For many years, the search for such species used their isolation from insect carcasses, followed by identification using conventional light or electron microscopy techniques. Thanks to the development of molecular methods, especially DNA sequencing and omics technologies, it is now possible to identify the most crucial EFs species and detect their presence in different ecological niches, including the soil or plant environments. EFs number around 1000 species [1816], the best-known being Aspergillus spp., Penicillium spp., Fusarium spp., and Acremonium spp. [1917]. Infection usually occurs through propagules that germinate and invade the host body after contact; the invasive mycelium then colonizses the host until it dies. Conidiation from emerging hyphae and/or the production of resting propagules follow the host death [2018]. Among the EFs primarily used for pest control, some Beauveria spp. (Hypocreales, Cordycipitaceae) are widely used against, for example, the coffee berry weevil Hypothenemus hampei (Ferrari, 1867) (Coleoptera, Curculionidae) [1715], the Asian corn borer Ostrinia furnacalis Guenée, 1854 (Lepidoptera, Crambidae), and the sweet potato weevil Cylas formicarius (Fabricius, 1798) (Coleoptera, Brentidae) [2119]. Many studies have deepened the knowledge about the role of Beauveria bassiana (Bals.) Vuill, 1912, as its insecticidal activity is due not only to the hyphae penetrating and spreading in the host body but also to the effect caused by various toxins [2220]. This fungus demonstrated its relevance in banana crops protection from Cosmopolites sordidus (Germar, 1824) (Coleoptera, Dryophthoridae) [2321],[2422],[2523] due to its ability to significantly reduce the weevil survival [2523]. Beauveria bassiana products are widely applied on banana plantations to manage C. sordidus and use pheromone for mass trapping. Another Beauveria species, Beauveria caledonica, is responsible for the lethal infections of C. sordidus in banana plantations in South America. This fungus produces various secondary metabolites and can modulate the pest immune response [2523],[2624]. Studies with Metarhizium anisopliae (Metschn.) Sorok, 1883 reported the potential of this fungus in controlling adult weevils [2725]. Several studies are underway to control P. spumarius, indicated as the main vector of the bacterium Xylella fastidiosa Wells, Raju et al., 1986 involved in the OQDS (Olive Quick Decline Syndrome) in the Salento Peninsula (southern Italy). The insect can acquire and inoculate the bacterium from/to different host plants [2826]; therefore, it is essential to limit the transmission of X. fastidiosa by managing its vector. Recent studies analyzse the ability of some Trichoderma spp. isolates in decreasing the survival of P. spumarius [2927]. An innovative IPM approach may include developing EF-based biocontrol actions. EF also represents an essential source of natural molecules capable of affecting P. spumarius metabolism and reproduction, thus limiting the pests’ indirect damage to plants [2927]. Species of the genus Trichoderma are among the most studied and used biocontrol agents worldwide. They not only produce benefits as plant growth promoters but also act, with various mechanisms, against other microorganisms in plant defensce. Volatile and non-volatile compounds produced by some species of Trichoderma can be perceived by the olfactory structures of P. spumarius [2927], modifying and directing the insect’s food preferences towards other areas of reduced agricultural interest [2927],[3028],[3129]. Although supported by valid research data, the information available in the literature on the exploitation of EFs as biocontrol agents still needs to be comprehensive. Critical data on the exploitation of EFs as practical means of biological control and information on the mechanisms involved in fungal-insect interactions still need to be included in many world regions. Therefore, efforts are still required to identify and characterizse new fungal strains to investigate their entomopathogenic capacity as an alternative to pesticides.3. Multitrophic Interactions of Entomopathogenic Fungi, Crops, and Insects
Insect pathogens were isolated from Mediterranean soils (Alicante, SE Spain) using Galleria mellonella L., 1758 (Lepidoptera, Pyralidae) larvae baits [3230]. Samples from 61 sites were from agroecosystems and forests, while soils under Nerium oleander L., 1753, gave results from natural environments and gardens. Entomopathogenic fungi (EFs) are the most frequent insect pathogens (32.8% soils). Beauveria bassiana is the most abundant species (21% soil). Metarhizium anisopliae (6.4%) and Akanthomyces lecanii (Zimm.) Spatafora, Kepler, and Shrestha, 2017 {Lecanicillium lecanii (Zimm.) Gams [=Verticillium lecanii Zimm.]} (4.8%) are less frequent. Beauveria bassiana also scored the highest virulence in a single soil sample (ca. 90% infected insects) and is the most frequent EF (77.8%) in soils under N. oleander. Soils from commercial crop fields of food security importance, such as bananas, are also reservoirs of EFs [3331]. Reports indicate that B. bassiana is a cosmopolitan entomopathogen, especially in warm areas [3432]. Economically important pests, such as thrips [3533], aphids [3634], or pine processionary (Thaumetopoea pityocampa [Denis and Schiffermüller, 1775] [Lepidoptera, Notodontidae]) [3735], were detected naturally infected with EFs. Beauveria bassiana (isolate Bb203) also infected adults of the Red Palm Weevil, Rhynchophorus ferrugineus Olivier, 1790 (RPW), in the field (palm groves) just at the first weevil introduction in south-eastern Spain [3836]. Beauveria bassiana 203 proved more pathogenic to R. ferrugineus than strains from other hosts and sources [3937]. The strain applied three times at three-month intervals to field palms naturally infested with RPW caused 70–85% insect mortality [4038]. Therefore, EFs are present in arid environments and have great potential for IPM of severe insect pests [4139],[4240]. EFs can also colonizse plants and plant waste. The latter is the most frequent component of soil organic matter. Evaluation of the growth and multiplication (conidiation) of common entomopathogens rises from inoculation (on almond peels) and gardening (palm waste) substrates obtained from Mediterranean ecosystems by-products of agriculture [4341]. The development of entomopathogens depends on the type of substrate. Akanthomyces lecanii grows and sporulates well on almond mesocarp, but Paecilomyces farinosus (Holmsk.) A.H.S.Br. and G.Sm., 1957 does not. Beauveria bassiana uses palm seed nutrients for growth and sporulation, and leaves of the Mediterranean dwarf palm Chamaerops humilis L., 1753 promote the growth and sporulation of both A. lecanii and B. bassiana. The date palm (Phoenix dactylifera L., 1753) has a mycobiota that includes-sporulating fungi (Penicillium spp. and Cladosporium spp.). Fusarium oxysporum Schltdl., 1824 saprotroph and an undescribed Lecanicillium c.f. psalliotae (Treschew) Zare and W. Gams, 2001 entomopathogen colonise leaves infested with Marlatt red-scale (Phoenicococcus marlatti Cockerell, 1899—Hemiptera, Phoenicococcidae) [4442]. Palm pathogens, entomopathogenic and saprotrophic fungi strongly interact with each other; B. bassiana strongly inhibits Penicillium vermoesenii [=Nalanthamala vermoesenii (Biourge) Schroers, 2005] (Figure 1), a fungal necrotrophy of palms.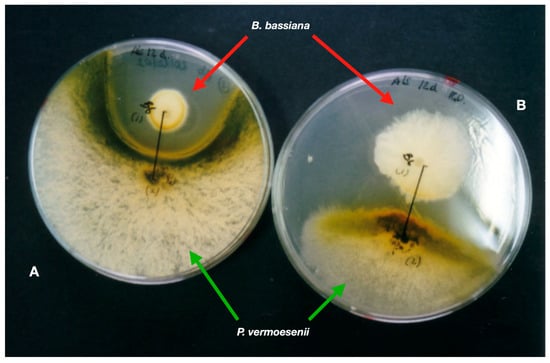
References
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al.et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484, 10.1126/science.1226727.
- Engel, M.S. Insect evolution. Current Biology 2015, 25, R868–R872, 10.1016/j.cub.2015.07.059 .
- Berenbaum, M.. Insect biodiversity—Millions and millions; Foottit, R.G.; Adler, P.H., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 783–792.
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The evolution of insect biodiversity. Current Biology 2021, 31, R1299–R1311, 10.1016/j.cub.2021.08.057 .
- Coll, M.; Guershon, M. Omnivory in Terrestrial Arthropods: Mixing Plant and Prey Diets. Annual Review of Entomology 2002, 47, 267-297, 10.1146/annurev.ento.47.091201.145209.de Bary, H.A.. Die Erscheinung der Symbiose: Vortrag, Gehalten auf der Versammlung Deutscher Naturforscher und Aerzte zu Cassel; Verlag von Karl J. Trübner: Strassburg, Germany, 1879; pp. 1–30.
- Price, P.W.; Denno, R.F.; Eubanks, M.D.; Finke, D.L.; Kaplan, I. . Insect Ecology: Behavior, Populations and Communities; Cambridge University Press: Cambridge, UK, 2011; pp. 307.Tellez, G. Prokaryotes Versus Eukaryotes: Who is Hosting Whom?. Frontiers in Veterinary Science 2014, 1, 3, 10.3389/fvets.2014.00003.
- de Bary, H.A.. Die Erscheinung der Symbiose: Vortrag, Gehalten auf der Versammlung Deutscher Naturforscher und Aerzte zu Cassel; Verlag von Karl J. Trübner: Strassburg, Germany, 1879; pp. 1–30.Hosokawa, T.; Fukatsu, T. Relevance of microbial symbiosis to insect behavior. Current Opinion in Insect Science 2020, 39, 91–100, 10.1016/j.cois.2020.03.004 .
- Tellez, G. Prokaryotes Versus Eukaryotes: Who is Hosting Whom?. FrDouglas, A.E. Mycetocyte symbiosis in insects. Biolontgiers in Veterinary Science 20cal Reviews 14, 1, 3, 10.3389/fvets.2014.00003.989, 64, 409–434, 10.1111/j.1469-185x.1989.tb00682.x .
- Hosokawa, T.; Fukatsu, T. Relevance of microbial symbiosis to insect behavior. CMoya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Naturrent Opinion in Insect Science Reviews Genetics 2020, 38, 9, 91–100, 10.1016/j.cois.2020.03.004 ., 218–229, 10.1038/nrg2319.
- Douglas, A.E. Mycetocyte symbiosis in insects. BEngel, P.; Moran, N.A. The gut microbiota of insects - diversity in structure and function. FEMS Microbiologiy Ecal Reviews ology 201989, 64, 409–434, 10.1111/j.1469-185x.1989.tb00682.x .3, 37, 699–735, 10.1111/1574-6976.12025 .
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: genomic insights into prokaryote-animal symbioses. NFerrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philosophical Transacture Reviews Geneticions of the Royal Society B: Biological Sciences 2008, 9, 218–229, 10.1038/nrg2319.11, 366, 1389–1400, 10.1098/rstb.2010.0226 .
- Engel, P.; Moran, N.A. The gut microbiota of insects - diversity in structure and function. FEMSFrago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Microbiology Ecology n Ecology & Evolution 2013, 32, 27, 699–735, 10.1111/1574-6976.12025 ., 705–711, 10.1016/j.tree.2012.08.013 .
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. PhLeung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vilosophe et Mical Transactions of the Royal Society B: Biological Sciences lieu/Life & Environment 20011, 366, 1389–1400, 10.1098/rstb.2010.0226 .8, 58, 107-115.
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. Bin Ecology & EvControlution 2012, 27, 705–711, 10.1016/j.tree.2012.08.013 .0, 55, 89–102, 10.1007/s10526-009-9238-5.
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. VieRoy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annual Ret Milieu/Life & Environment view of Entomology 2008, 6, 58, 107-115.1, 331–357, 10.1146/annurev.ento.51.110104.150941.
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. BSt. Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied MicrobioClontrgy and Biotechnol ogy 2010, , 855, 89–102, 10.1007/s10526-009-9238-5., 901–907, 10.1007/s00253-009-2306-z.
- Roy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. AnnuGurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biological Review Cof Entomntrology 20106, , 551, 331–357, 10.1146/annurev.ento.51.110104.150941., 34–41, 10.1016/j.biocontrol.2010.06.011.
- St. Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. AppPosada-Flórez, F.J. Production of Beauveria bassiana fungal spores on rice to control the coffee berry borer, Hypothenemus hampei, in Colombia. Journalied Microbiology and Biotechnology of Insect Science 2010, 8, 85, 901–907, 10.1007/s00253-009-2306-z., 41, 10.1673/031.008.4101.
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. BYasuda, K. Auto-infection system for the sweet potato weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) with entomopathogenic fungi, Beauveria bassiana using a modified sex pheromone trap in the field. Applied Entological Contrmology and Zool 20ogy 10, 55, 34–41, 10.1016/j.biocontrol.2010.06.011.999, 34, 501–505.
- Posada-Flórez, F.J. Production of Beauveria bassiana fungal spores on rice to control the coffee berry borer, Hypothenemus hampei, in Colombia. Kumar, V.; Singh, G.P.; Babu, A.M.; Ahsan, M.M.; Datta, R.K. Germination, penetration, and invasion of Beauveria bassiana on silkworm, Bombyx mori, causing white muscardine. Italian Journal of Insect Science 2008, 8, 41, 10.1673/031.008.4101.Zoology 1999, 66, 39–43, 10.1080/11250009909356235.
- Yasuda, K. Auto-infection system for the sweet potato weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) with entomopathogenic fungi, Beauveria bassiana using a modified sex pheromone trap in the field. ApplGodonou, I.; Green, K.R.; Oduro, K.A.; Lomer, C.J.; Afreh-Nuamah, K. Field evaluation of selected formulations of Beauveria bassiana for the management of the banana weevil (Cosmopolites sordidus) on plantain (Musa spp., AAB group). Bied Eocontomology and Zorol Science and Technology 1999, 34, 501–505. 2000, 10, 779–788, 10.1080/09583150020011726.
- Kumar, V.; Singh, G.P.; Babu, A.M.; Ahsan, M.M.; Datta, R.K. Germination, penetration, and invasion of Beauveria bassiana on silkworm, Bombyx mori, causing white muscardine. ITinzaara, W.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. Biocontarolian Journal of Zoo Science and Technology 1999, 66, 39–43, 10.1080/11250009909356235. 2007, 17, 111–124, 10.1080/09583150600937089.
- Godonou, I.; Green, K.R.; Oduro, K.A.; Lomer, C.J.; Afreh-Nuamah, K. Field evaluation of selected formulations of Beauveria bassiana for the management of the banana weevil (Cosmopolites sordidus) on plantain (Musa spp., AAB group). Fancelli, M.; Batista Dias, A.; Delalibera Júnior, I.; Cerqueira de Jesus, S.; Souza do Nascimento, A.; de Oliveira e Silva, S.; Correa Caldas, R.; da Silva Ledo, C.A. Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in plantain. BiocontrolMed Science and TechnoResearch Internationalogy 2000, 13, 2010, 779–788, 10.1080/09583150020011726.3, 184756, 10.1155/2013/184756 .
- Tinzaara, W.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. BiocoMc Namara, L.; Dolan, S.K.; Walsh, J.M.D.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Funtrogal Science and TechnBiology 2007, 19, 17, 111–124, 10.1080/09583150600937089.23, 601–610, 10.1016/j.funbio.2019.01.004.
- Fancelli, M.; Batista Dias, A.; Delalibera Júnior, I.; Cerqueira de Jesus, S.; Souza do Nascimento, A.; de Oliveira e Silva, S.; Correa Caldas, R.; da Silva Ledo, C.A. Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in plantain. BMembang, G.; Ambang, Z.; Mahot, H.C.; Kuate, A.F.; Fiaboe, K.K.M.; Hanna, R. Cosmopolites sordidus (Germar) susceptibility to indigenous Cameroonian Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) isolates. Journal of ApplioMed Research InternationaEntomol ogy 202013, 20, 13, 184756, 10.1155/2013/184756 .44, 468–480, 10.1111/jen.12757.
- Mc Namara, L.; Dolan, S.K.; Walsh, J.M.D.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. FCornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. Journgal Bial of Applied Entomology 2019, 7, 14123, 601–610, 10.1016/j.funbio.2019.01.004., 80–87, 10.1111/jen.12365.
- Membang, G.; Ambang, Z.; Mahot, H.C.; Kuate, A.F.; Fiaboe, K.K.M.; Hanna, R. Cosmopolites sordidus (Germar) susceptibility to indigenous Cameroonian Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) isolates. JGerminara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLournalS of Applied ONEntomology 2020, 17, 144, 468–480, 10.1111/jen.12757.2, e0190454, 10.1371/journal.pone.0190454.
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. JGanassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long chain alcohols produced by Trichoderma citrinoviride have phagodeterrent activity against the bird cherry-oat aphid Rhopalosiphum padi. Frournal of Applied Entomtiers in Microbiology 2017, 141, 80–87, 10.1111/jen.12365.6, 7, 297, 10.3389/fmicb.2016.00297 .
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLEvidente, A.; Andolfi, A.; Cimmino, A.; Ganassi, S.; Altomare, C.; Favilla, M.; De Cristofaro, A.; Vitagliano, S.; Sabatini, A.M. Bisorbicillinoids produced by the fungus Trichoderma citrinoviride affect feeding preference of the aphid Schizaphis graminum. Journal oSf Chemical ONE cology 20017, 12, e0190454, 10.1371/journal.pone.0190454.9, 35, 533–541, 10.1007/s10886-009-9632-6.
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long chain alcohols produced by Trichoderma citrinoviride have phagodeterrent activity against the bird cherry-oat aphid Rhopalosiphum padi. FroAsensio, L.; Carbonell, T.; López-Jiménez, J.A.; Lopez-Llorca, L.V. Entomopathogenic fungi in soils from Alicante province [Spain]. Spantiers in Microbiology sh Journal of Agricultural Research 20016, 7, 297, 10.3389/fmicb.2016.00297 .3, 1, 9, 10.5424/sjar/2003013-33.
- Evidente, A.; Andolfi, A.; Cimmino, A.; Ganassi, S.; Altomare, C.; Favilla, M.; De Cristofaro, A.; Vitagliano, S.; Sabatini, A.M. Bisorbicillinoids produced by the fungus Trichoderma citrinoviride affect feeding preference of the aphid Schizaphis graminum. JourLozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Inal of Chsemical Ecology cts 20209, 35, 533–541, 10.1007/s10886-009-9632-6., 11, 509, 10.3390/insects11080509.
- Asensio, L.; Carbonell, T.; López-Jiménez, J.A.; Lopez-Llorca, L.V. Entomopathogenic fungi in soils from Alicante province [Spain]. SpanZimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Bish Jocournal of Agricultural Researntrol Science and Tech nology 2003, 7, 1, 9, 10.5424/sjar/2003013-33.7, 553–596, 10.1080/09583150701309006.
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). InsecLopez-Llorca, L.V. Thrips affected by entomopathogenic fungus Beauveria bassiana. Phytoma Es 2020, 11, 509, 10.3390/insects11080509.paña 1993, 53, 41–43.
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. BioLopez-Llorca, L.V. Aphid infection by the entomopathogen Erynia neoaphidis—SEM study. Mycontrol Science and Technology 2007, 1ogist 1993, 7, 553–596, 10.1080/09583150701309006., 166–168, 10.1016/S0269-915X(09)80387-X.
- Lopez-Llorca, L.V. Thrips affected by entomopathogenic fungus Beauveria bassiana. PhLopez-Llorca, L.V.; Carbonell, T.; Gomez-Vidal, S. Degradation of insect cuticle by Paecilomyces farinosus proteases. Mytcoloma Egical Progresspaña 1993, 53, 41–43. 2002, 1, 249–256, 10.1007/s11557-006-0022-y.
- Lopez-Llorca, L.V. Aphid infection by the entomopathogen Erynia neoaphidis—SEM study. Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. Myicologist roscopy Research and Technique 201993, 0, 7, 166–168, 10.1016/S0269-915X(09)80387-X.3, 714–725, 10.1002/jemt.20812.
- Lopez-Llorca, L.V.; Carbonell, T.; Gomez-Vidal, S. Degradation of insect cuticle by Paecilomyces farinosus proteases. MycoRicaño, J.; Güerri-Agulló, B.; Serna-Sarriás, M.J.; Rubio-Llorca, G.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Flogrical Progress da Entomologist 2002, 1, 249–256, 10.1007/s11557-006-0022-y.13, 96, 1311–1324, 10.1653/024.096.0410.
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. MicrGüerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Floscopy Research and Technique ida Entomologist 20110, 73, 714–725, 10.1002/jemt.20812., 94, 737–747, 10.1653/024.094.0402.
- Ricaño, J.; Güerri-Agulló, B.; Serna-Sarriás, M.J.; Rubio-Llorca, G.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. FlAguilera Sammaritano, J.A.; López Lastra, C.C.; Leclerque, A.; Vazquez, F.; Toro, M.E.; D’Alessandro, C.P.; Cuthbertson, A.G.S.; Lechner, B.E. Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. Biocontrida Entomologist ol Science and Technology 2013, 96, 26, 1311–1324, 10.1653/024.096.0410., 1668–1682, 10.1080/09583157.2016.1231776.
- Güerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. FlCasique-Valdés, R.; Torres-Acosta, R.I.; Sánchez-Peña, S.R. Metarhizium acridum and other entomopathogenic fungi from grasshop- pers at arid sites of northeastern Mexico. Southwesteridan Entomologist 2011, 922, 4, 737–747, 10.1653/024.094.0402.7, 547–558, 10.3958/059.047.0302.
- Aguilera Sammaritano, J.A.; López Lastra, C.C.; Leclerque, A.; Vazquez, F.; Toro, M.E.; D’Alessandro, C.P.; Cuthbertson, A.G.S.; Lechner, B.E. Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. BLopez-Llorca, L.V.; Carbonell, T.; Salinas, J. Colonization of plant waste substrates by entomopathogenic and mycoparasitic fungi—A SEM study. Miocontrol Scieronce and Technology 20 16, 26, 1668–1682, 10.1080/09583157.2016.1231776.999, 30, 325–333, 10.1016/S0968-4328(99)00031-1.
- Casique-Valdés, R.; Torres-Acosta, R.I.; Sánchez-Peña, S.R. Metarhizium acridum and other entomopathogenic fungi from grasshop- pers at arid sites of northeastern Mexico. SouthwAsensio, L.; López-Jiménez, J.Á.; López-Llorca, L.V. Mycobiota of the date palm phylloplane: description and interactions. Revistern Entomologist a Iberoamericana de Micología 2022, 07, 247, 547–558, 10.3958/059.047.0302., 299–304, 10.1016/s1130-1406(07)70060-8 .
- Lopez-Llorca, L.V.; Carbonell, T.; Salinas, J. Colonization of plant waste substrates by entomopathogenic and mycoparasitic fungi—A SEM study. Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 1999, 2006, 30, 325–333, 10.1016/S0968-4328(99)00031-1.7, 624–632, 10.1016/j.micron.2006.02.003.
- Asensio, L.; López-Jiménez, J.Á.; López-Llorca, L.V. Mycobiota of the date palm phylloplane: description and interactions. Mohamed Mahmoud, F.; Krimi, Z.; Maciá-Vicente, J.G.; Brahim Errahmani, M.; Lopez-Llorca, L.V. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Revista Iberoamericana de Micología 20017, 2, 34, 299–304, 10.1016/s1130-1406(07)70060-8 ., 116–120, 10.1016/j.riam.2016.06.007.
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. Journal of Applied Microbion logy 2006, 21, 137, 624–632, 10.1016/j.micron.2006.02.003.0, 570–581, 10.1111/jam.14503.
- Mohamed Mahmoud, F.; Krimi, Z.; Maciá-Vicente, J.G.; Brahim Errahmani, M.; Lopez-Llorca, L.V. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nature Reviewsta Iberoamericana de Micología Microbiology 2017, 34, 116–120, 10.1016/j.riam.2016.06.007.3, 11, 789–799, 10.1038/nrmicro3109.
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. Journal of Applied Aranda-Martinez,A.; NaranjoOrtiz,M.Á.; AbihssiraGarcía,I.S.; Zavala-Gonzalez,E.A.; Lopez-Llorca,L.V. Ethanol production from chitosan by the nematophagous fungus Pochonia chlamydosporia and the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Microbiology ical Research 2021, 137, 20, 570–581, 10.1111/jam.14503.4, 30–39, 10.1016/j.micres.2017.07.009.
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. NaGonzález-Mas,N.; Gutiérrez-Sánchez,F.; Sánchez-Ortiz,A.; Grandi,L.; Turlings,T.C.J.; ManuelMuñoz-Redondo,J.; Moreno- Rojas, J.M.; Quesada-Moraga, E. Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. Fronture Reviews Microbiology rs in Plant Science 20213, , 11, 789–799, 10.1038/nrmicro3109.2, 660460, 10.3389/fpls.2021.660460.
- Aranda-Martinez,A.; NaranjoOrtiz,M.Á.; AbihssiraGarcía,I.S.; Zavala-Gonzalez,E.A.; Lopez-Llorca,L.V. Ethanol production from chitosan by the nematophagous fungus Pochonia chlamydosporia and the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. MRivas-Franco,F.; Hampton,J.G.; Altier,N.A.; Swaminathan,J.; Rostás,M.; Wessman,P.; Saville,D.J.; Jackson,T.A.; Jackson,M.A.; Glare, T.R.; et al. Production of microsclerotia from entomopathogenic fungi and use in maize seed coating as delivery for biocontrol against Fusarium graminearum. Fronticerobiological Res in Sustainable Food Systemsearch 202017, 20, 4, 30–39, 10.1016/j.micres.2017.07.009., 606828, 10.3389/fsufs.2020.606828.
- González-Mas,N.; Gutiérrez-Sánchez,F.; Sánchez-Ortiz,A.; Grandi,L.; Turlings,T.C.J.; ManuelMuñoz-Redondo,J.; Moreno- Rojas, J.M.; Quesada-Moraga, E. Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. FMathulwe, L.L.; Malan, A.P.; Stokwe, N.F. Mass production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against insect pests. Journal ontiers in Plant Science f Visualized Experiments 2021, 2, 1812, 660460, 10.3389/fpls.2021.660460., e63246, 10.3791/63246.
- Rivas-Franco,F.; Hampton,J.G.; Altier,N.A.; Swaminathan,J.; Rostás,M.; Wessman,P.; Saville,D.J.; Jackson,T.A.; Jackson,M.A.; Glare, T.R.; et al. Production of microsclerotia from entomopathogenic fungi and use in maize seed coating as delivery for biocontrol against Fusarium graminearum. FGómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrontiphorers in Susistainable Food Systems 2020, 4, 606828, 10.3389/fsufs.2020.606828.9, 9, 2996–3005, 10.1002/elps.200900192.
- Mathulwe, L.L.; Malan, A.P.; Stokwe, N.F. Mass production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against insect pests. JPusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycournal of Visualized Experiments ogy 2022, 181, e63246, 10.3791/63246.18, 9, 189–201, 10.1080/21501203.2018.1473299.
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. EPalma-Guerrero, J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N.D. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genectrophoresis tics and Biology 2009, 9, 2996–3005, 10.1002/elps.200900192., 46, 585–594, 10.1016/j.fgb.2009.02.010.
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. International Journal of Myolecology ular Sciences 2018, 9, 189–201, 10.1080/21501203.2018.1473299.9, 20, 332, 10.3390/ijms20020332.
- Palma-Guerrero, J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N.D. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Lopez-Nuñez, R.; Suarez-Fernandez, M.; Lopez-Moya, F.; Lopez-Llorca, L.V. Chitosan and nematophagous fungi for sustainable management of nematode pests. Furongal Genetics andtiers in Fungal Biology 2009, 46, 585–594, 10.1016/j.fgb.2009.02.010.22, 3, 980341, 10.3389/ffunb.2022.980341.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. International Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan: Role of fungal cell wall on sensitivity to chitosan. Journal of Basic Molecular Sciences icrobiology 2019, 20, 332, 10.3390/ijms20020332.6, 56, 1059–1070, 10.1002/jobm.201500775.
- Lopez-Nuñez, R.; Suarez-Fernandez, M.; Lopez-Moya, F.; Lopez-Llorca, L.V. Chitosan and nematophagous fungi for sustainable management of nematode pests. FLopez-Moya, F.; Lopez-Llorca, L.V. Omics for investigating chitosan as an antifungal and gene modulator. Jourontiers in Fungal Bal of Fungiology 2022, 3, 980341, 10.3389/ffunb.2022.980341.16, 2, 11, 10.3390/jof2010011.
- Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan: Role of fungal cell wall on sensitivity to chitosan. JoEscudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Furnal of Basic Microbiogal Biology 2016, 56, 1059–1070, 10.1002/jobm.201500775., 120, 572–585, 10.1016/j.funbio.2015.12.005.
- Lopez-Moya, F.; Lopez-Llorca, L.V. Omics for investigating chitosan as an antifungal and gene modulator. JEscudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Frountiernal of Fungi s in Plant Science 2016, 2, 11, 10.3390/jof2010011.7, 8, 1415, 10.3389/fpls.2017.01415.
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. FuSuarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environmengtal BMicrobiology 20216, 1, 20, 572–585, 10.1016/j.funbio.2015.12.005.3, 4980–4997, 10.1111/1462-2920.15408.
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. FrontLin, R.; Qin, F.; Shen, B.; Shi, Q.; Liu, C.; Zhang, X.; Jiao, Y.; Lu, J.; Gao, Y.; Suarez-Fernandez, M.; et al. Genome and secretome analysis of Pochonia chlamydosporia provide new insight into egg-parasitic mechanisms. Sciers in Plant Scienctific Re ports 2017, 8, 8, 1415, 10.3389/fpls.2017.01415., 1123, 10.1038/s41598-018-19169-5.
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. EnviJalinas, J.; Guerri-Agullo, B.; Mankin, R.W.; Lopez-Follana, R.; Lopez-Llorca, L.V. Acoustic assessment of Beauveria Bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. Jouronmental Microbial of Economic Entomology 2021, 23, 4980–4997, 10.1111/1462-2920.15408.5, 108, 444–453, 10.1093/jee/tov023.
- Lin, R.; Qin, F.; Shen, B.; Shi, Q.; Liu, C.; Zhang, X.; Jiao, Y.; Lu, J.; Gao, Y.; Suarez-Fernandez, M.; et al. Genome and secretome analysis of Pochonia chlamydosporia provide new insight into egg-parasitic mechanisms. ScieJalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journtial of Fungic Reports 2018, 22, 8, 1123, 10.1038/s41598-018-19169-5., 843, 10.3390/jof8080843.
- Jalinas, J.; Guerri-Agullo, B.; Mankin, R.W.; Lopez-Follana, R.; Lopez-Llorca, L.V. Acoustic assessment of Beauveria Bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of EcoFunomgic Entomology 2015, 1022, 8, 444–453, 10.1093/jee/tov023., 843, 10.3390/jof8080843.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.
