Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Camila Xu and Version 3 by Camila Xu.
The influence between microorganisms and insects has led to the establishment of different types of interaction that can be summarised in various forms of interactions. De Bary first defined symbiosis as “the cohabitation of distinct organisms”. Symbiosis is here intended as a significant biological liaison between or among species. Symbiosis is one of the leading evolutionary drivers promoting natural biological novelty. Symbiotic relationships between prokaryotes and eukaryotes are present in all kingdoms of life.
- alien
- invasive or quarantine pest
- Integrated Farming
- resilience
1. Introduction
Insects have inhabited the Earth for approximately 480 million years, representing the dominant life form as species and biomass [1],[2],[3],[4]. Their presence expresses the high biodiversity of insects in a wide range of ecological niches and results from their genetic plasticity, adaptability, and co-evolutionary processes with other organisms.
The influence between microorganisms and insects has led to the establishment of different types of interaction that can be summarised in various forms of interactions. De Bary [5] first defined symbiosis as “the cohabitation of distinct organisms”. Symbiosis is here intended as a significant biological liaison between or among species. Symbiosis is one of the leading evolutionary drivers promoting natural biological novelty. Symbiotic relationships between prokaryotes and eukaryotes are present in all kingdoms of life [6]].
Symbioses between two organisms can be broadly classified as mutualism, commensalism, or antagonism, depending on the interaction between the species involved. The impact of the symbiont on the amphitryon can highlight an evolutionary continuum between antagonism (negative interaction), commensalism (neutral interaction), and mutualism (beneficial interaction) [7]. Symbiotic relationships drive many interesting biological processes, both within individuals and at the ecological system level.
For the sake of this contribution, researchers consider ectosymbiont as the guest living out of the host body and endosymbiont as a guest living in the host body. Inside the amphitryon body, the guest may live within specialised host cells [8][9][10].
Mutualism provides an advantage for both species involved. Mutualistic microorganisms can give the host essential nutrients, protection from enemies, increase fitness, and mediate the host’s interaction with other species [11]. Mutualists can be divided into obligate and facultative. Obligate or primary symbionts are microorganisms necessary for the host’s survival. They tend to improve the nutritional aspects of unbalanced diets on which the host feed [12]. Facultative symbionts are not essential for the survival of their host and have broader effects ranging from modifying nutritional aspects to manipulating reproduction. Secondary mutualists can often only be found in a fraction of the host population [11][12].
Commensal symbiosis represents a symbiotic relationship in which one organism benefits without associated costs [12][13].
Finally, parasitism or antagonism represents an unbalanced interaction in favour of the guest microorganism that takes advantage of the insect, generating a loss of fitness or causing host death. Antagonists may be obligate (host-specific) or facultative generalists. Antagonism between insects and entomopathogenic organisms results from co-evolution in which the pathogen aims to host exploitation better and improve its transmission. In contrast, the insect seeks to exclude the pathogen more effectively by improving its defence strategies [[14]]. Both actors involved in antagonism adopt physiological, ecological, and ethological adaptations to maximise their fitness [15].
 EFs (B. bassiana, Lecanicillium dimorphum (J.D.Chen) Zare and W.Gams, 2001, and Lecanicillium c.f. psalliotae) artificially inoculated in living plants act as true endophytes [43]; fungi survive and spread in date palm (P. dactylifera) petiole tissues (parenchyma and vascular tissue) at least 30 days after inoculation. Beauveria bassiana is a natural endophyte from date palm roots [44]. This fungus was isolated from the roots of date palms in two coastal dune sites with high and low human impact in south-eastern Spain. Root colonisation by endophytic insect-pathogenic fungi has recently been reviewed [45]. Root and microbiota respiration [46] depletes oxygen in the rhizosphere. Fungal parasites of invertebrates, such as the nematophagous Pochonia chlamydosporia (Goddard) Zare and W. Gams, 2001 or the entomopathogens B. bassiana and M. anisopliae, breach chitin-rich barriers to infect the host. These biocontrol fungi can also ferment chitosan, a chitin derivative [47]. Apart from their application in biofuel production, this trait can be an adaptation for survival and insect infection by EFs in the rhizosphere. Entomopathogenic fungi are part of phylloplane and rhizosphere mycobiomes. Their endophytic behaviour allows them to colonise plant-derived substrates, affecting plant-volatile emissions during insect infestations [48]. Plant-derived substrates, such as rice grains, can be used for mass production and formulation of EFs [49][50].
Based on previous reports (see above) on the endophytic behaviour of EFs, several studies tested the response of palms to inoculation with these biocontrol fungi. Beauveria bassiana, L. dimorphum, and L. cf. psalliotae induced proteins in plant defence or stress response [51]. The plant immune system responds to microbe-associated molecular patterns (MAMPs) derived from conserved structures (i.e., cell walls) of plant pathogens such as chitin [52]. Chitosan can permeabilise the membrane and kill plant pathogens such as bacteria and fungi in its deacetylated form [53]. EFs and nematophagous fungi (NFs) are compatible with chitosan since they have evolved low-fluidity membranes [54][55] and branched cell walls rich in β-1,3-glucan [56]. Moreover, EFs and NFs are in contact with chitin during host (insects and nematodes, respectively) infection. Chitosan modifies the transcriptome and biology of fungi and plants, causing cell stress [57]. Chitosan can enhance the pathogenicity of fungal parasites of nematode eggs [58][59][60]. These are close relatives of EFs, such as Metarhizium spp. [61]. Tests will explain the effect of chitosan on the EFs’ pathogenicity.
Acoustics reveals that RPW larvae have briefer movement and feeding activity with B. bassiana infection [62]. Researchers also have evidence that B. bassiana formulates used for RPW biocontrol in the field [38] repel adults of this insect pest [63]. Evidence suggested investigating entomopathogenic fungi and close fungal pathogens of invertebrates for volatiles capable of modifying the behaviour of insects of economic importance, such as weevils. Entomopathogenic fungi and close relative nematophagous fungi (Pochonia spp. egg parasites) emit volatile organic compounds (VOCs) capable of repelling C. sordidus [31] and RPW [64]. P201930831 and P202230103 insect repellents patented VOCs are on field trial for efficacy.
Finally, EFs are a component of plant and soil microbiomes. They are efficient insect pathogens with a multitrophic lifestyle, including plant endophytism, inducing plant defences and modifying insect pest behaviour with their VOCs, which work as low environmental impact tools for insect pest management.
EFs (B. bassiana, Lecanicillium dimorphum (J.D.Chen) Zare and W.Gams, 2001, and Lecanicillium c.f. psalliotae) artificially inoculated in living plants act as true endophytes [43]; fungi survive and spread in date palm (P. dactylifera) petiole tissues (parenchyma and vascular tissue) at least 30 days after inoculation. Beauveria bassiana is a natural endophyte from date palm roots [44]. This fungus was isolated from the roots of date palms in two coastal dune sites with high and low human impact in south-eastern Spain. Root colonisation by endophytic insect-pathogenic fungi has recently been reviewed [45]. Root and microbiota respiration [46] depletes oxygen in the rhizosphere. Fungal parasites of invertebrates, such as the nematophagous Pochonia chlamydosporia (Goddard) Zare and W. Gams, 2001 or the entomopathogens B. bassiana and M. anisopliae, breach chitin-rich barriers to infect the host. These biocontrol fungi can also ferment chitosan, a chitin derivative [47]. Apart from their application in biofuel production, this trait can be an adaptation for survival and insect infection by EFs in the rhizosphere. Entomopathogenic fungi are part of phylloplane and rhizosphere mycobiomes. Their endophytic behaviour allows them to colonise plant-derived substrates, affecting plant-volatile emissions during insect infestations [48]. Plant-derived substrates, such as rice grains, can be used for mass production and formulation of EFs [49][50].
Based on previous reports (see above) on the endophytic behaviour of EFs, several studies tested the response of palms to inoculation with these biocontrol fungi. Beauveria bassiana, L. dimorphum, and L. cf. psalliotae induced proteins in plant defence or stress response [51]. The plant immune system responds to microbe-associated molecular patterns (MAMPs) derived from conserved structures (i.e., cell walls) of plant pathogens such as chitin [52]. Chitosan can permeabilise the membrane and kill plant pathogens such as bacteria and fungi in its deacetylated form [53]. EFs and nematophagous fungi (NFs) are compatible with chitosan since they have evolved low-fluidity membranes [54][55] and branched cell walls rich in β-1,3-glucan [56]. Moreover, EFs and NFs are in contact with chitin during host (insects and nematodes, respectively) infection. Chitosan modifies the transcriptome and biology of fungi and plants, causing cell stress [57]. Chitosan can enhance the pathogenicity of fungal parasites of nematode eggs [58][59][60]. These are close relatives of EFs, such as Metarhizium spp. [61]. Tests will explain the effect of chitosan on the EFs’ pathogenicity.
Acoustics reveals that RPW larvae have briefer movement and feeding activity with B. bassiana infection [62]. Researchers also have evidence that B. bassiana formulates used for RPW biocontrol in the field [38] repel adults of this insect pest [63]. Evidence suggested investigating entomopathogenic fungi and close fungal pathogens of invertebrates for volatiles capable of modifying the behaviour of insects of economic importance, such as weevils. Entomopathogenic fungi and close relative nematophagous fungi (Pochonia spp. egg parasites) emit volatile organic compounds (VOCs) capable of repelling C. sordidus [31] and RPW [64]. P201930831 and P202230103 insect repellents patented VOCs are on field trial for efficacy.
Finally, EFs are a component of plant and soil microbiomes. They are efficient insect pathogens with a multitrophic lifestyle, including plant endophytism, inducing plant defences and modifying insect pest behaviour with their VOCs, which work as low environmental impact tools for insect pest management.
2. Interactions between Entomopathogenic Fungi and Pests
Biological control of invasive pests also relies on certain entomopathogenic fungi (EFs) that can infect hosts in agroecosystems and appear suitable for plant protection exploitation. For many years, the search for such species used their isolation from insect carcasses, followed by identification using conventional light or electron microscopy techniques. Thanks to the development of molecular methods, especially DNA sequencing and omics technologies, it is now possible to identify the most crucial EFs species and detect their presence in different ecological niches, including the soil or plant environments. EFs number around 1000 species [16], the best-known being Aspergillus spp., Penicillium spp., Fusarium spp., and Acremonium spp. [17]. Infection usually occurs through propagules that germinate and invade the host body after contact; the invasive mycelium then colonises the host until it dies. Conidiation from emerging hyphae and/or the production of resting propagules follow the host death [18]. Among the EFs primarily used for pest control, some Beauveria spp. (Hypocreales, Cordycipitaceae) are widely used against, for example, the coffee berry weevil Hypothenemus hampei (Ferrari, 1867) (Coleoptera, Curculionidae) [15], the Asian corn borer Ostrinia furnacalis Guenée, 1854 (Lepidoptera, Crambidae), and the sweet potato weevil Cylas formicarius (Fabricius, 1798) (Coleoptera, Brentidae) [19]. Many studies have deepened the knowledge about the role of Beauveria bassiana (Bals.) Vuill, 1912, as its insecticidal activity is due not only to the hyphae penetrating and spreading in the host body but also to the effect caused by various toxins [20]. This fungus demonstrated its relevance in banana crops protection from Cosmopolites sordidus (Germar, 1824) (Coleoptera, Dryophthoridae) [21][22][23] due to its ability to significantly reduce the weevil survival [23]. Beauveria bassiana products are widely applied on banana plantations to manage C. sordidus and use pheromone for mass trapping. Another Beauveria species, Beauveria caledonica, is responsible for the lethal infections of C. sordidus in banana plantations in South America. This fungus produces various secondary metabolites and can modulate the pest immune response [23][24]. Studies with Metarhizium anisopliae (Metschn.) Sorok, 1883 reported the potential of this fungus in controlling adult weevils [25]. Several studies are underway to control P. spumarius, indicated as the main vector of the bacterium Xylella fastidiosa Wells, Raju et al., 1986 involved in the OQDS (Olive Quick Decline Syndrome) in the Salento Peninsula (southern Italy). The insect can acquire and inoculate the bacterium from/to different host plants [26]; therefore, it is essential to limit the transmission of X. fastidiosa by managing its vector. Recent studies analyse the ability of some Trichoderma spp. isolates in decreasing the survival of P. spumarius [27]. An innovative IPM approach may include developing EF-based biocontrol actions. EF also represents an essential source of natural molecules capable of affecting P. spumarius metabolism and reproduction, thus limiting the pests’ indirect damage to plants [27]. Species of the genus Trichoderma are among the most studied and used biocontrol agents worldwide. They not only produce benefits as plant growth promoters but also act, with various mechanisms, against other microorganisms in plant defence. Volatile and non-volatile compounds produced by some species of Trichoderma can be perceived by the olfactory structures of P. spumarius [27], modifying and directing the insect’s food preferences towards other areas of reduced agricultural interest [27][28][29]. Although supported by valid research data, the information available in the literature on the exploitation of EFs as biocontrol agents still needs to be comprehensive. Critical data on the exploitation of EFs as practical means of biological control and information on the mechanisms involved in fungal-insect interactions still need to be included in many world regions. Therefore, efforts are still required to identify and characterise new fungal strains to investigate their entomopathogenic capacity as an alternative to pesticides.3. Multitrophic Interactions of Entomopathogenic Fungi, Crops, and Insects
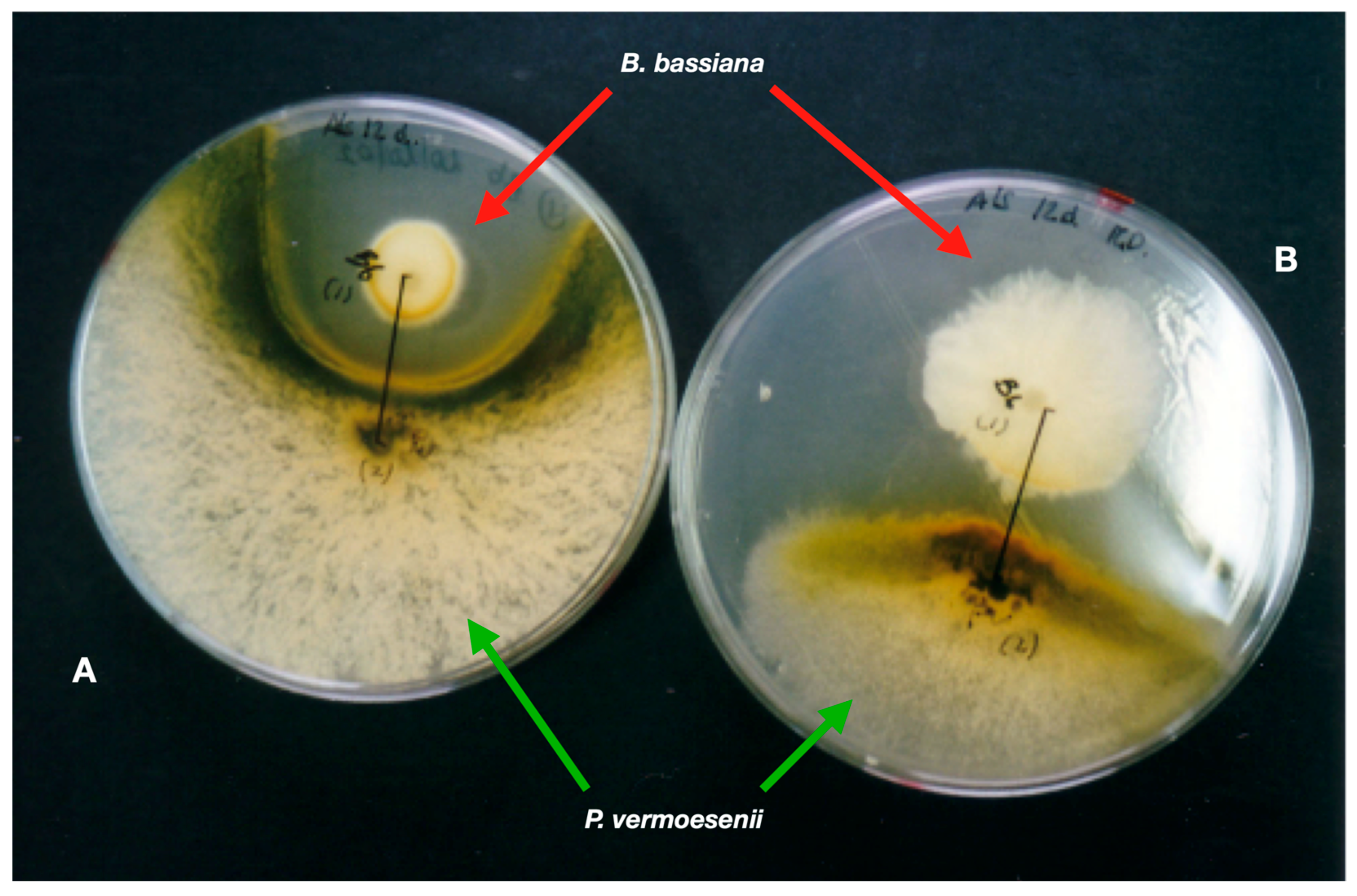
Insect pathogens were isolated from Mediterranean soils (Alicante, SE Spain) using Galleria mellonella L., 1758 (Lepidoptera, Pyralidae) larvae baits [30]. Samples from 61 sites were from agroecosystems and forests, while soils under Nerium oleander L., 1753, gave results from natural environments and gardens. Entomopathogenic fungi (EFs) are the most frequent insect pathogens (32.8% soils). Beauveria bassiana is the most abundant species (21% soil). Metarhizium anisopliae (6.4%) and Akanthomyces lecanii (Zimm.) Spatafora, Kepler and Shrestha, 2017 {Lecanicillium lecanii (Zimm.) Gams [=Verticillium lecanii Zimm.]} (4.8%) are less frequent. Beauveria bassiana also scored the highest virulence in a single soil sample (ca. 90% infected insects) and is the most frequent EF (77.8%) in soils under N. oleander. Soils from commercial crop fields of food security importance, such as bananas, are also reservoirs of EFs [31]. Reports indicate that B. bassiana is a cosmopolitan entomopathogen, especially in warm areas [32]. Economically important pests, such as thrips [33], aphids [34], or pine processionary (Thaumetopoea pityocampa [Denis and Schiffermüller, 1775] [Lepidoptera, Notodontidae]) [35], were detected naturally infected with EFs. Beauveria bassiana (isolate Bb203) also infected adults of the Red Palm Weevil, Rhynchophorus ferrugineus Olivier, 1790 (RPW), in the field (palm groves) just at the first weevil introduction in south-eastern Spain [36]. Beauveria bassiana 203 proved more pathogenic to R. ferrugineus than strains from other hosts and sources [37]. The strain applied three times at three-month intervals to field palms naturally infested with RPW caused 70–85% insect mortality [38]. Therefore, EFs are present in arid environments and have great potential for IPM of severe insect pests [39][40]. EFs can also colonise plants and plant waste. The latter is the most frequent component of soil organic matter. Evaluation of the growth and multiplication (conidiation) of common entomopathogens rises from inoculation (on almond peels) and gardening (palm waste) substrates obtained from Mediterranean ecosystems by-products of agriculture [41]. The development of entomopathogens depends on the type of substrate. Akanthomyces lecanii grows and sporulates well on almond mesocarp, but Paecilomyces farinosus (Holmsk.) A.H.S.Br. and G.Sm., 1957 does not. Beauveria bassiana uses palm seed nutrients for growth and sporulation, and leaves of the Mediterranean dwarf palm Chamaerops humilis L., 1753 promote the growth and sporulation of both A. lecanii and B. bassiana. The date palm (Phoenix dactylifera L., 1753) has a mycobiota that includes-sporulating fungi (Penicillium spp. and Cladosporium spp.). Fusarium oxysporum Schltdl., 1824 saprotroph and an undescribed Lecanicillium c.f. psalliotae (Treschew) Zare and W. Gams, 2001 entomopathogen colonise leaves infested with Marlatt red-scale (Phoenicococcus marlatti Cockerell, 1899—Hemiptera, Phoenicococcidae) [42]. Palm pathogens, entomopathogenic and saprotrophic fungi strongly interact with each other; B. bassiana strongly inhibits Penicillium vermoesenii [=Nalanthamala vermoesenii (Biourge) Schroers, 2005] (Figure 1), a fungal necrotrophy of palms.
Figure 1. Beauveria bassiana (red arrows) inhibits the fungus palm pathogen Penicillium vermoesenii (green arrows). (A) Both fungi interact directly on the PDA medium. (B) The same two fungi on top of a dialysis membrane overlaid onto PDA.
References
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al.et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484, 10.1126/science.1226727.
- Engel, M.S. Insect evolution. Current Biology 2015, 25, R868–R872, 10.1016/j.cub.2015.07.059 .
- Berenbaum, M.. Insect biodiversity—Millions and millions; Foottit, R.G.; Adler, P.H., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 783–792.
- Tihelka, E.; Cai, C.; Giacomelli, M.; Lozano-Fernandez, J.; Rota-Stabelli, O.; Huang, D.; Engel, M.S.; Donoghue, P.C.J.; Pisani, D. The evolution of insect biodiversity. Current Biology 2021, 31, R1299–R1311, 10.1016/j.cub.2021.08.057 .
- de Bary, H.A.. Die Erscheinung der Symbiose: Vortrag, Gehalten auf der Versammlung Deutscher Naturforscher und Aerzte zu Cassel; Verlag von Karl J. Trübner: Strassburg, Germany, 1879; pp. 1–30.
- Tellez, G. Prokaryotes Versus Eukaryotes: Who is Hosting Whom?. Frontiers in Veterinary Science 2014, 1, 3, 10.3389/fvets.2014.00003.
- Hosokawa, T.; Fukatsu, T. Relevance of microbial symbiosis to insect behavior. Current Opinion in Insect Science 2020, 39, 91–100, 10.1016/j.cois.2020.03.004 .
- Douglas, A.E. Mycetocyte symbiosis in insects. Biological Reviews 1989, 64, 409–434, 10.1111/j.1469-185x.1989.tb00682.x .
- Moya, A.; Peretó, J.; Gil, R.; Latorre, A. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nature Reviews Genetics 2008, 9, 218–229, 10.1038/nrg2319.
- Engel, P.; Moran, N.A. The gut microbiota of insects - diversity in structure and function. FEMS Microbiology Ecology 2013, 37, 699–735, 10.1111/1574-6976.12025 .
- Ferrari, J.; Vavre, F. Bacterial symbionts in insects or the story of communities affecting communities. Philosophical Transactions of the Royal Society B: Biological Sciences 2011, 366, 1389–1400, 10.1098/rstb.2010.0226 .
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect-plant interactions. Trends in Ecology & Evolution 2012, 27, 705–711, 10.1016/j.tree.2012.08.013 .
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie et Milieu/Life & Environment 2008, 58, 107-115.
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. BioControl 2010, 55, 89–102, 10.1007/s10526-009-9238-5.
- Roy, H.E.; Steinkraus, D.C.; Eilenberg, J.; Hajek, A.E.; Pell, J.K. Bizarre interactions and endgames: Entomopathogenic fungi and their arthropod hosts. Annual Review of Entomology 2006, 51, 331–357, 10.1146/annurev.ento.51.110104.150941.
- St. Leger, R.J.; Wang, C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Applied Microbiology and Biotechnology 2010, 85, 901–907, 10.1007/s00253-009-2306-z.
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biological Control 2010, 55, 34–41, 10.1016/j.biocontrol.2010.06.011.
- Posada-Flórez, F.J. Production of Beauveria bassiana fungal spores on rice to control the coffee berry borer, Hypothenemus hampei, in Colombia. Journal of Insect Science 2008, 8, 41, 10.1673/031.008.4101.
- Yasuda, K. Auto-infection system for the sweet potato weevil, Cylas formicarius (Fabricius) (Coleoptera: Curculionidae) with entomopathogenic fungi, Beauveria bassiana using a modified sex pheromone trap in the field. Applied Entomology and Zoology 1999, 34, 501–505.
- Kumar, V.; Singh, G.P.; Babu, A.M.; Ahsan, M.M.; Datta, R.K. Germination, penetration, and invasion of Beauveria bassiana on silkworm, Bombyx mori, causing white muscardine. Italian Journal of Zoology 1999, 66, 39–43, 10.1080/11250009909356235.
- Godonou, I.; Green, K.R.; Oduro, K.A.; Lomer, C.J.; Afreh-Nuamah, K. Field evaluation of selected formulations of Beauveria bassiana for the management of the banana weevil (Cosmopolites sordidus) on plantain (Musa spp., AAB group). Biocontrol Science and Technology 2000, 10, 779–788, 10.1080/09583150020011726.
- Tinzaara, W.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. Biocontrol Science and Technology 2007, 17, 111–124, 10.1080/09583150600937089.
- Fancelli, M.; Batista Dias, A.; Delalibera Júnior, I.; Cerqueira de Jesus, S.; Souza do Nascimento, A.; de Oliveira e Silva, S.; Correa Caldas, R.; da Silva Ledo, C.A. Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in plantain. BioMed Research International 2013, 2013, 184756, 10.1155/2013/184756 .
- Mc Namara, L.; Dolan, S.K.; Walsh, J.M.D.; Stephens, J.C.; Glare, T.R.; Kavanagh, K.; Griffin, C.T. Oosporein, an abundant metabolite in Beauveria caledonica, with a feedback induction mechanism and a role in insect virulence. Fungal Biology 2019, 123, 601–610, 10.1016/j.funbio.2019.01.004.
- Membang, G.; Ambang, Z.; Mahot, H.C.; Kuate, A.F.; Fiaboe, K.K.M.; Hanna, R. Cosmopolites sordidus (Germar) susceptibility to indigenous Cameroonian Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) isolates. Journal of Applied Entomology 2020, 144, 468–480, 10.1111/jen.12757.
- Cornara, D.; Cavalieri, V.; Dongiovanni, C.; Altamura, G.; Palmisano, F.; Bosco, D.; Porcelli, F.; Almeida, R.P.P.; Saponari, M. Transmission of Xylella fastidiosa by naturally infected Philaenus spumarius (Hemiptera, Aphrophoridae) to different host plants. Journal of Applied Entomology 2017, 141, 80–87, 10.1111/jen.12365.
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, e0190454, 10.1371/journal.pone.0190454.
- Ganassi, S.; Grazioso, P.; De Cristofaro, A.; Fiorentini, F.; Sabatini, M.A.; Evidente, A.; Altomare, C. Long chain alcohols produced by Trichoderma citrinoviride have phagodeterrent activity against the bird cherry-oat aphid Rhopalosiphum padi. Frontiers in Microbiology 2016, 7, 297, 10.3389/fmicb.2016.00297 .
- Evidente, A.; Andolfi, A.; Cimmino, A.; Ganassi, S.; Altomare, C.; Favilla, M.; De Cristofaro, A.; Vitagliano, S.; Sabatini, A.M. Bisorbicillinoids produced by the fungus Trichoderma citrinoviride affect feeding preference of the aphid Schizaphis graminum. Journal of Chemical Ecology 2009, 35, 533–541, 10.1007/s10886-009-9632-6.
- Asensio, L.; Carbonell, T.; López-Jiménez, J.A.; Lopez-Llorca, L.V. Entomopathogenic fungi in soils from Alicante province [Spain]. Spanish Journal of Agricultural Research 2003, 1, 9, 10.5424/sjar/2003013-33.
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509, 10.3390/insects11080509.
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Science and Technology 2007, 17, 553–596, 10.1080/09583150701309006.
- Lopez-Llorca, L.V. Thrips affected by entomopathogenic fungus Beauveria bassiana. Phytoma España 1993, 53, 41–43.
- Lopez-Llorca, L.V. Aphid infection by the entomopathogen Erynia neoaphidis—SEM study. Mycologist 1993, 7, 166–168, 10.1016/S0269-915X(09)80387-X.
- Lopez-Llorca, L.V.; Carbonell, T.; Gomez-Vidal, S. Degradation of insect cuticle by Paecilomyces farinosus proteases. Mycological Progress 2002, 1, 249–256, 10.1007/s11557-006-0022-y.
- Güerri-Agulló, B.; Gómez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Infection of the red palm weevil (Rhynchophorus ferrugineus) by the entomopathogenic fungus Beauveria bassiana: a SEM study. Microscopy Research and Technique 2010, 73, 714–725, 10.1002/jemt.20812.
- Ricaño, J.; Güerri-Agulló, B.; Serna-Sarriás, M.J.; Rubio-Llorca, G.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Evaluation of the pathogenicity of multiple isolates of Beauveria bassiana (Hypocreales: Clavicipitaceae) on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) for the assessment of a solid formulation under simulated field conditions. Florida Entomologist 2013, 96, 1311–1324, 10.1653/024.096.0410.
- Güerri-Agulló, B.; López-Follana, R.; Asensio, L.; Barranco, P.; Lopez-Llorca, L.V. Use of a solid formulation of Beauveria bassiana for biocontrol of the red palm weevil (Rhynchophorus ferrugineus) (Coleoptera: Dryophthoridae) under field conditions in SE Spain. Florida Entomologist 2011, 94, 737–747, 10.1653/024.094.0402.
- Aguilera Sammaritano, J.A.; López Lastra, C.C.; Leclerque, A.; Vazquez, F.; Toro, M.E.; D’Alessandro, C.P.; Cuthbertson, A.G.S.; Lechner, B.E. Control of Bemisia tabaci by entomopathogenic fungi isolated from arid soils in Argentina. Biocontrol Science and Technology 2016, 26, 1668–1682, 10.1080/09583157.2016.1231776.
- Casique-Valdés, R.; Torres-Acosta, R.I.; Sánchez-Peña, S.R. Metarhizium acridum and other entomopathogenic fungi from grasshop- pers at arid sites of northeastern Mexico. Southwestern Entomologist 2022, 47, 547–558, 10.3958/059.047.0302.
- Lopez-Llorca, L.V.; Carbonell, T.; Salinas, J. Colonization of plant waste substrates by entomopathogenic and mycoparasitic fungi—A SEM study. Micron 1999, 30, 325–333, 10.1016/S0968-4328(99)00031-1.
- Asensio, L.; López-Jiménez, J.Á.; López-Llorca, L.V. Mycobiota of the date palm phylloplane: description and interactions. Revista Iberoamericana de Micología 2007, 24, 299–304, 10.1016/s1130-1406(07)70060-8 .
- Gómez-Vidal, S.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron 2006, 37, 624–632, 10.1016/j.micron.2006.02.003.
- Mohamed Mahmoud, F.; Krimi, Z.; Maciá-Vicente, J.G.; Brahim Errahmani, M.; Lopez-Llorca, L.V. Endophytic fungi associated with roots of date palm (Phoenix dactylifera) in coastal dunes. Revista Iberoamericana de Micología 2017, 34, 116–120, 10.1016/j.riam.2016.06.007.
- Hu, S.; Bidochka, M.J. Root colonization by endophytic insect-pathogenic fungi. Journal of Applied Microbiology 2021, 130, 570–581, 10.1111/jam.14503.
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nature Reviews Microbiology 2013, 11, 789–799, 10.1038/nrmicro3109.
- Aranda-Martinez,A.; NaranjoOrtiz,M.Á.; AbihssiraGarcía,I.S.; Zavala-Gonzalez,E.A.; Lopez-Llorca,L.V. Ethanol production from chitosan by the nematophagous fungus Pochonia chlamydosporia and the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana. Microbiological Research 2017, 204, 30–39, 10.1016/j.micres.2017.07.009.
- González-Mas,N.; Gutiérrez-Sánchez,F.; Sánchez-Ortiz,A.; Grandi,L.; Turlings,T.C.J.; ManuelMuñoz-Redondo,J.; Moreno- Rojas, J.M.; Quesada-Moraga, E. Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. Frontiers in Plant Science 2021, 12, 660460, 10.3389/fpls.2021.660460.
- Rivas-Franco,F.; Hampton,J.G.; Altier,N.A.; Swaminathan,J.; Rostás,M.; Wessman,P.; Saville,D.J.; Jackson,T.A.; Jackson,M.A.; Glare, T.R.; et al. Production of microsclerotia from entomopathogenic fungi and use in maize seed coating as delivery for biocontrol against Fusarium graminearum. Frontiers in Sustainable Food Systems 2020, 4, 606828, 10.3389/fsufs.2020.606828.
- Mathulwe, L.L.; Malan, A.P.; Stokwe, N.F. Mass production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against insect pests. Journal of Visualized Experiments 2022, 181, e63246, 10.3791/63246.
- Gómez-Vidal, S.; Salinas, J.; Tena, M.; Lopez-Llorca, L.V. Proteomic analysis of date palm (Phoenix dactylifera L.) responses to endophytic colonization by entomopathogenic fungi. Electrophoresis 2009, 9, 2996–3005, 10.1002/elps.200900192.
- Pusztahelyi, T. Chitin and chitin-related compounds in plant–fungal interactions. Mycology 2018, 9, 189–201, 10.1080/21501203.2018.1473299.
- Palma-Guerrero, J.; Huang, I.-C.; Jansson, H.-B.; Salinas, J.; Lopez-Llorca, L.V.; Read, N.D. Chitosan permeabilizes the plasma membrane and kills cells of Neurospora crassa in an energy dependent manner. Fungal Genetics and Biology 2009, 46, 585–594, 10.1016/j.fgb.2009.02.010.
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. International Journal of Molecular Sciences 2019, 20, 332, 10.3390/ijms20020332.
- Lopez-Nuñez, R.; Suarez-Fernandez, M.; Lopez-Moya, F.; Lopez-Llorca, L.V. Chitosan and nematophagous fungi for sustainable management of nematode pests. Frontiers in Fungal Biology 2022, 3, 980341, 10.3389/ffunb.2022.980341.
- Aranda-Martinez, A.; Lopez-Moya, F.; Lopez-Llorca, L.V. Cell wall composition plays a key role on sensitivity of filamentous fungi to chitosan: Role of fungal cell wall on sensitivity to chitosan. Journal of Basic Microbiology 2016, 56, 1059–1070, 10.1002/jobm.201500775.
- Lopez-Moya, F.; Lopez-Llorca, L.V. Omics for investigating chitosan as an antifungal and gene modulator. Journal of Fungi 2016, 2, 11, 10.3390/jof2010011.
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biology 2016, 120, 572–585, 10.1016/j.funbio.2015.12.005.
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan increases tomato root colonization by Pochonia chlamydosporia and their combination reduces root-knot nematode damage. Frontiers in Plant Science 2017, 8, 1415, 10.3389/fpls.2017.01415.
- Suarez-Fernandez, M.; Sambles, C.; Lopez-Moya, F.; Nueda, M.J.; Studholme, D.J.; Lopez-Llorca, L.V. Chitosan modulates Pochonia chlamydosporia gene expression during nematode egg parasitism. Environmental Microbiology 2021, 23, 4980–4997, 10.1111/1462-2920.15408.
- Lin, R.; Qin, F.; Shen, B.; Shi, Q.; Liu, C.; Zhang, X.; Jiao, Y.; Lu, J.; Gao, Y.; Suarez-Fernandez, M.; et al. Genome and secretome analysis of Pochonia chlamydosporia provide new insight into egg-parasitic mechanisms. Scientific Reports 2018, 8, 1123, 10.1038/s41598-018-19169-5.
- Jalinas, J.; Guerri-Agullo, B.; Mankin, R.W.; Lopez-Follana, R.; Lopez-Llorca, L.V. Acoustic assessment of Beauveria Bassiana (Hypocreales: Clavicipitaceae) effects on Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) larval activity and mortality. Journal of Economic Entomology 2015, 108, 444–453, 10.1093/jee/tov023.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.
- Jalinas, J.; Lopez-Moya, F.; Marhuenda-Egea, F.C.; Lopez-Llorca, L.V. Beauveria bassiana (Hypocreales: Clavicipitaceae) volatile organic compounds (VOCs) repel Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Journal of Fungi 2022, 8, 843, 10.3390/jof8080843.
More
