
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joao Gomes | -- | 1349 | 2022-12-21 10:26:14 | | | |
| 2 | Peter Tang | Meta information modification | 1349 | 2022-12-21 10:31:31 | | |
Video Upload Options
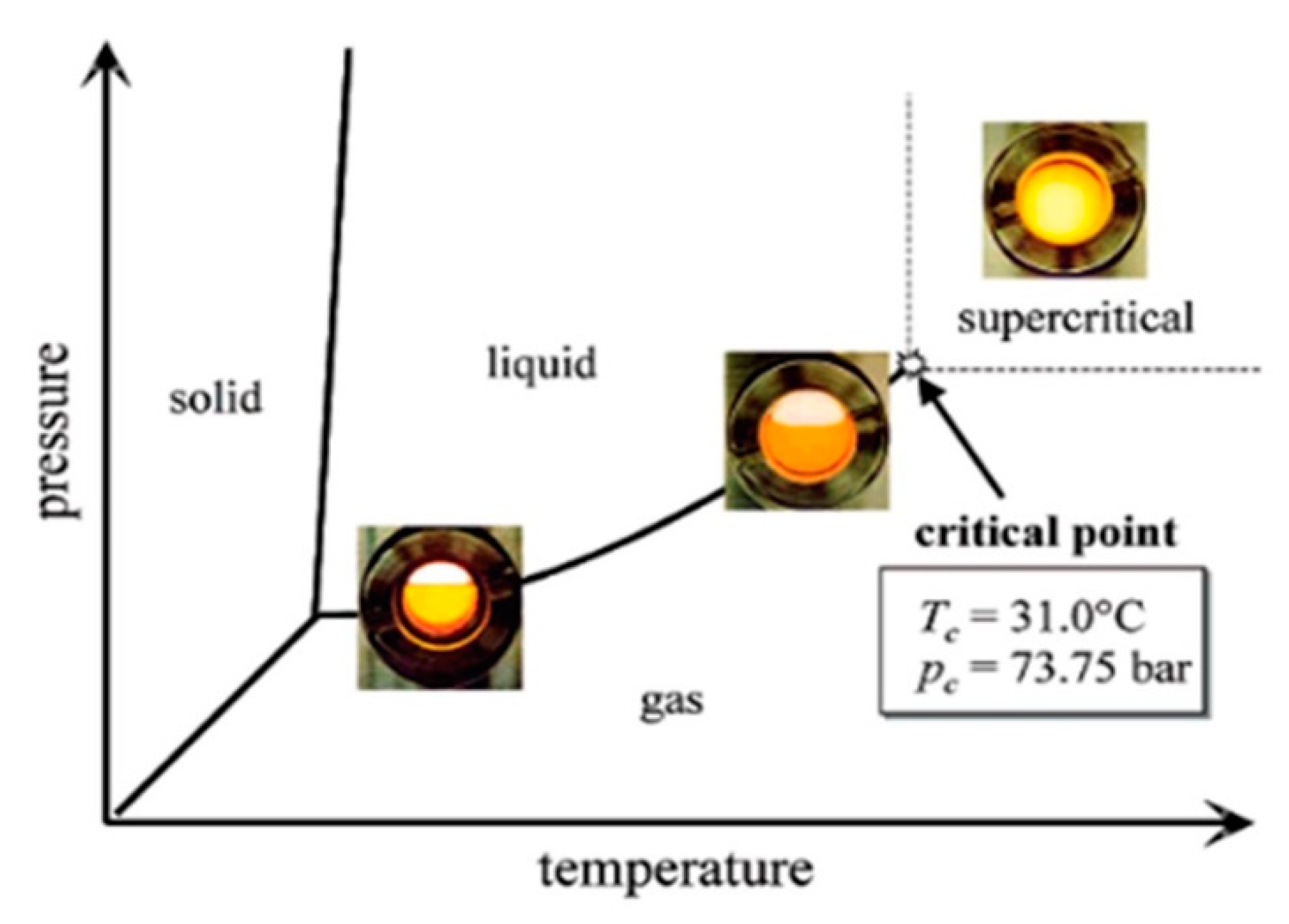
In an era where sustainability is becoming the main driving force for research and development, supercritical fluids-based techniques are presented as a very efficient alternative technology to conventional extraction, purification, and recrystallization processes. Supercritical antisolvent (SAS) precipitation is a novel technique that can replace liquid antisolvent precipitation techniques. Additionally, through the optimization of precipitation operating conditions, morphology, particle size, and particle size distribution of nanoparticles can be controlled. As an antisolvent, supercritical carbon dioxide (scCO2) is far more sustainable than its conventional liquid counterparts; not only does it have a critical point (304 K and 73.8 bar) on its phase diagram that allows for the precipitation processes to be developed so close to room temperature, but also its recovery and, consequently, the precipitated solute purification stage is considerably simpler. This technique can be used efficiently for preparing nanocatalysts to be used in biodiesel production processes.
1. Introduction
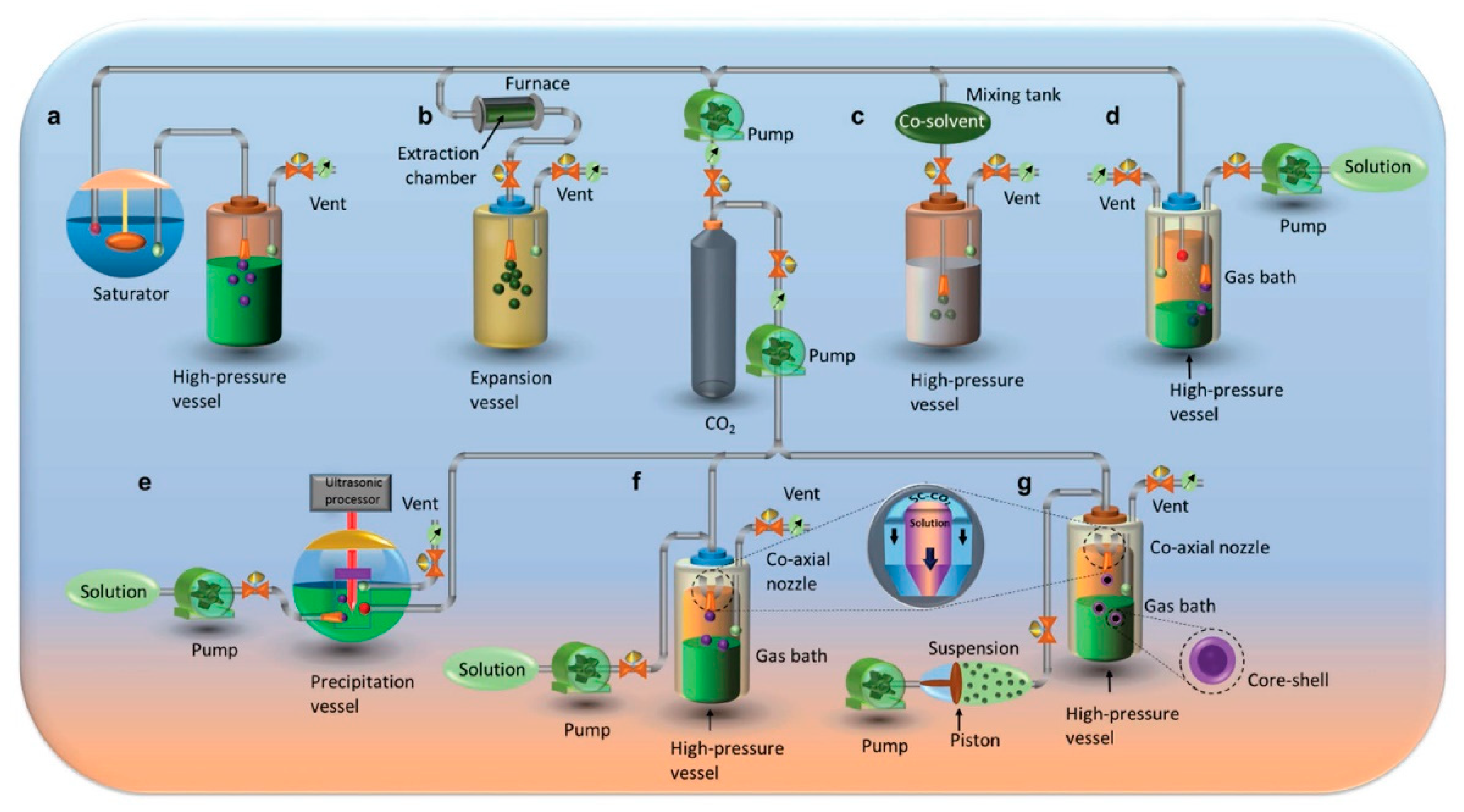
2. Preparation of Nanomaterials Using Supercritical CO2
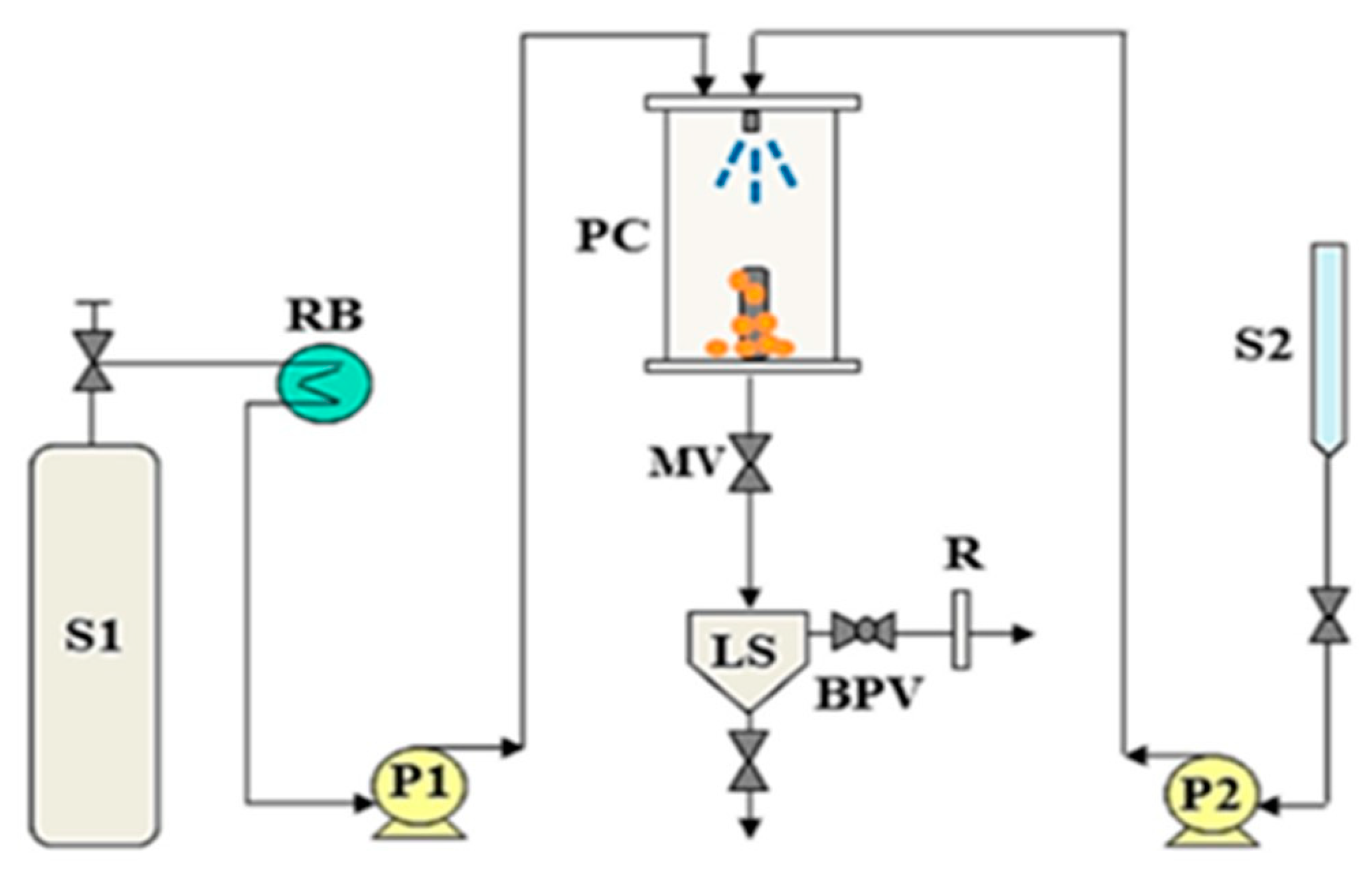
3. Supercritical Antisolvent (SAS) as a Micronization Technique
References
- Su, W.; Zhang, H.; Xing, Y.; Li, X.; Wang, J.; Cai, C. A Bibliometric Analysis and Review of Supercritical Fluids for the Synthesis of Nanomaterials. Nanomaterials 2021, 11, 336.
- Knez; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial Applications of Supercritical Fluids: A Review. Energy 2014, 77, 235–243.
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are Supercritical Fluids Solvents for the Future? Chem. Eng. Process. Process Intensif. 2019, 141, 107532.
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils–A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260.
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic Acid–PVP Nanostructured Composite Microparticles by Supercritical Antisolvent Precipitation. Chem. Eng. J. 2015, 277, 286–294.
- Skouta, R. Selective Chemical Reactions in Supercritical Carbon Dioxide, Water, and Ionic Liquids. Green Chem. Lett. Rev. 2009, 2, 121–156.
- Liu, H.; Chen, B.Q.; Pan, Y.J.; Fu, C.P.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Role of Supercritical Carbon Dioxide (ScCO2) in Fabrication of Inorganic-Based Materials: A Green and Unique Route. Sci. Technol. Adv. Mater. 2021, 22, 695–717.
- Li, K.; Xu, Z. A Review of Current Progress of Supercritical Fluid Technologies for E-Waste Treatment. J. Clean. Prod. 2019, 227, 794–809.
- West, C. Recent Trends in Chiral Supercritical Fluid Chromatography. TrAC Trends Anal. Chem. 2019, 120, 115648.
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342.
- Leitner, W. Supercritical Carbon Dioxide as a Green Reaction Medium for Catalysis. Acc. Chem. Res. 2002, 35, 746–756.
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410.
- Banković–Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of Nano CaO–Based Catalysts in Biodiesel Synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760.
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent Advancement in Biodiesel Production Methodologies Using Various Feedstock: A Review. Renew. Sustain. Energy Rev. 2018, 90, 356–369.
- Rajput, N. Methods of Preparation of Nanoparticles-A Review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811.
- Santos, S.; Puna, J.; Gomes, J.; Marchetti, J. A Review on the Use of Bio/Nanostructured Heterogeneous Catalysts in Biodiesel Production. In Nano-and Biocatalysts for Biodiesel Production; Wiley: Hoboken, NJ, USA, 2021; pp. 59–91.
- Reverchon, E. Supercritical Antisolvent Precipitation of Micro- and Nano-Particles. J. Supercrit. Fluids 1999, 15, 1–21.
- Franco, P.; Marco, I. De Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938.
- Reverchon, E.; Della Porta, G. Production of Antibiotic Micro- and Nano-Particles by Supercritical Antisolvent Precipitation. Powder Technol. 1999, 106, 23–29.
- Majerik, V.; Charbit, G.; Badens, E.; Horváth, G.; Szokonya, L.; Bosc, N.; Teillaud, E. Bioavailability Enhancement of an Active Substance by Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2007, 40, 101–110.
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical Carbon Dioxide-Based Technologies for the Production of Drug Nanoparticles/Nanocrystals–A Comprehensive Review. Adv. Drug Deliv. Rev. 2018, 131, 22–78.
- Da Silva, E.P.; Winkler, M.E.G.; Giufrida, W.M.; Cardozo-Filho, L.; Alonso, C.G.; Lopes, J.B.O.; Rubira, A.F.; Silva, R. Effect of Phase Composition on the Photocatalytic Activity of Titanium Dioxide Obtained from Supercritical Antisolvent. J. Colloid Interface Sci. 2019, 535, 245–254.
- De Marco, I. The Supercritical Antisolvent Precipitation from a Sustainable Perspective: A Life Cycle Assessment. J. CO2 Util. 2022, 55, 101808.
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 16, 1700433.
- Franco, P.; De Marco, I. Nanoparticles and Nanocrystals by Supercritical CO2-Assisted Techniques for Pharmaceutical Applications: A Review. Appl. Sci. 2021, 11, 1476.
- Tenorio, A.; Gordillo, D.M.; Pereyra, M.C.; Martínez de la Ossa, E.J. Relative Importance of the Operating Conditions Involved in the Formation of Nanoparticles of Ampicillin by Supercritical Antisolvent Precipitation. Ind. Eng. Chem. Res. 2006, 46, 114–123.
- Mahdi Pourmortazavi, S.; Somayyeh Hajimirsadeghi, S. Application of Supercritical Carbon Dioxide in Energetic Materials Processes: A Review. Ind. Eng. Chem. Res. 2005, 44, 6523–6533.
- Reverchon, E.; Della Porta, G.; Di Trolio, A.; Pace, S. Supercritical Antisolvent Precipitation of Nanoparticles of Superconductor Precursors. Ind. Eng. Chem. Res. 1998, 37, 952–958.
- Tang, Z.R.; Edwards, J.K.; Bartley, J.K.; Taylor, S.H.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Nanocrystalline Cerium Oxide Produced by Supercritical Antisolvent Precipitation as a Support for High-Activity Gold Catalysts. J. Catal. 2007, 249, 208–219.
- Reverchon, E.; Adami, R.; Caputo, G.; De Marco, I. Spherical Microparticles Production by Supercritical Antisolvent Precipitation: Interpretation of Results. J. Supercrit. Fluids 2008, 47, 70–84.
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle Formation of Lycopene/β-Cyclodextrin Inclusion Complex Using Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2013, 83, 97–103.
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Antisolvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346.




