Ever since Baron Charles de la Tour first theorized supercritical fluids (SCFs) in 1822 [
1,
2,
3], several research studies have been executed regarding its applications, resulting in several technologies such as supercritical fluid extraction, supercritical drying, supercritical dyeing, and supercritical fluid chromatography. [
1] SCFs are characterized by having both their temperature and pressure values higher than their critical point, where a significant difference between liquid and gas does not exist [
2,
4].
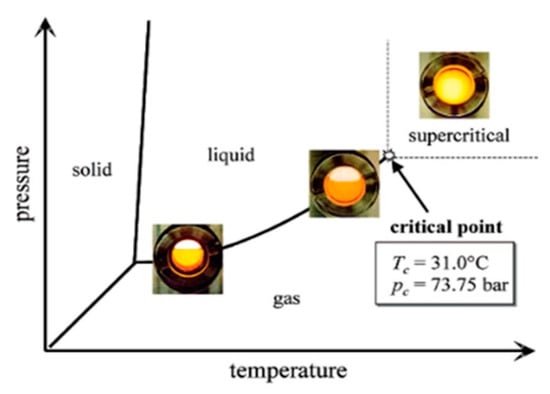
As these operating conditions exceed the critical point, a clear interface between the liquid and gas phases tends to disappear and, thus, becomes a mixed gas, as shown in
Figure 1. This mixed gas has properties inherited from both gaseous and liquid states, namely: low viscosity, high density, high diffusivity, non-existing surface tension, good fluidity, heat and mass transfer characteristics, as well as an adjustable solvent selectivity [
1,
5,
6,
7,
8,
9,
10].
2. Preparation of Nanomaterials Using Supercritical CO2
Nanoscale materials, often known as nanomaterials, are defined by the International Union of Pure and Applied Chemistry (IUPAC) as having organized components with at least one dimension less than 100 nm [
16]. New nanomaterials, including nanoclays, nanofibers, nanoporous materials, carbon nanotubes, nanocomposites, and nanoparticles, have recently been used for several different applications [
17].
The most used methods to produce nanomaterials are gas condensation, vacuum evaporation and deposition, precipitation, impregnation, chemical vapor deposition, nano-grinding, calcination-hydration-dehydration, and sol–gel techniques [
1,
18,
19,
20]. Liquid anti-solvent processes are also used in the industry, based on the miscibility between two solvents. The solute to be micronized has to be soluble in the first solvent, but not soluble in the anti-solvent. Therefore, by adding the anti-solvent, the formation of a solution between the two liquids and the supersaturation is induced, and subsequent precipitation of the solute occurs [
21]. This traditional micronization technique usually produces wide particle size ranges and products with an uneven morphology. The elimination of liquid solvent residues is also a matter of concern [
21,
22]. These limitations can be particularly pertinent for some industrial applications, such as the production of pharmaceutical compounds [
23].
Due to the features of supercritical fluids (SCFs) that have been previously described, approaches based on SCFs have been suggested as an alternative to traditional procedures [
5]. By adjusting the operational parameters like the temperature, pressure, and solvent flow rate, supercritical fluids like scCO
2 can produce nanoparticles. It is also feasible to modify the particle size as well as the morphology of nanoparticles by utilizing the unique properties of supercritical solvents [
1]. The earliest evidence of supercritical fluids being used for particle formation upon their depressurization was found in 1879 by Hannay and Hogart [
24,
25]. Surprisingly, the first patent for the rapid expansion of supercritical expansion solutions by valves or other spraying devices, or Rapid Expansion of Supercritical Solutions (RESS), was not published until 1986, more than a century after the invention was made [
25]. Since CO
2 has a low critical pressure, and particularly temperature, in addition to being abundant and reasonably non-expensive, it has several technological advantages: used CO
2 can be easily collected and reutilized [
26], and different processes have been developed for that purpose.
Recently, new proposals and developments have been made concerning various micronization technologies aiming to benefit from the peculiarities of fluids at supercritical conditions: new approaches for particle formation methods focused on the use of scCO
2 have started to be designed, developed, and tested, such as solvent (RESS), antisolvent (supercritical antisolvent (SAS)), aerosol solvent extraction system (ASES), precipitation with compressed antisolvent (PCA), gaseous antisolvent (GAS), supercritical antisolvent using enhanced mass transfer (SAS-EM), solution enhanced dispersion by supercritical fluids (SEDS), suspension-enhanced dispersion by supercritical fluids (SpEDS)), co-solute (particle formation from gas-saturated solutions (PGSS)) and co-solvent [
25,
27,
28,
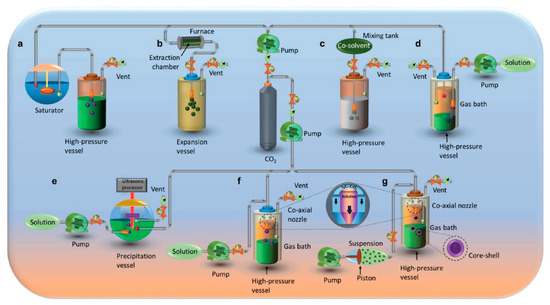
29]. A conceptual picture of the various scCO
2-based particle production processes is shown in
Figure 2.
Figure 2. Conceptually distinct particle precipitation mechanisms are represented using scCO
2: (
a) Formation of particles from gas-saturated solutions (PGSS). (
b) Expansion (rapid) of supercritical solutions (RESS). (
c) Expansion (rapid) of a supercritical solution into a liquid solvent (RESOLV). (
d) Precipitation using a compressed anti-solvent (PCA)/Aerosol solvent extraction system (ASES). (
e) Supercritical antisolvent with enhanced mass transfer (SAS-EM). (
f) Solution-enhanced dispersion by supercritical fluids (SEDS). (
g) Suspension-enhanced dispersion by supercritical fluids (SpEDS) [
28].
3. Supercritical Antisolvent (SAS) as a Micronization Technique
Supercritical antisolvent precipitation (SAS), is a novel, ecologically benign method of creating nanomaterials that may be used as a substitute for liquid solvent precipitation since it is far more efficient. scCO
2 has been extensively used for producing a variety of materials, such as polymers, biopolymers, superconductors, explosives, colouring agents, active pharmaceutical ingredients (APIs), and catalysts, using an antisolvent for the controlled precipitation of solids dissolved in conventional solvent, if the processed compounds do not dissolve in the supercritical medium [
5,
23,
30,
31,
32,
33,
34].
Additionally, the size and morphology of the resulting solids precipitated by SAS technologies are usually correlated with the system solvent/antisolvent high-pressure VLEs (vapour-liquid equilibrium), or the position of the SAS operating point around the critical point of the mixture (MCP) [
7,
29]. For instance, if the process is being conducted as a single-phase method with no interface between the solution and the antisolvent, this indicates that micronization is occurring at supercritical conditions, i.e., above the MCP, and the very fast diffusion of scCO
2 into the liquid solvent causes its expansion, thus producing the solute’s supersaturation and resulting in forming nanoparticle morphologies that are not typically achieved by traditional catalyst preparation methods [
26,
33]. This phenomenon, adding to the quasi-zero surface tension of scCO
2, allows for obtaining particles of smaller size and having a narrow particle size distribution (PSD) with the complete elimination of the solvents, when compared to the traditional micronization techniques [
23,
31,
36,
37]; or, if the process shows a two-phase mixing, the micronization is occurring at subcritical operation conditions, i.e., in the biphasic region below the MCP, resulting in the production of microparticles [
25,
29].
The success of SAS precipitation techniques is strongly dependant on the affinity between the solvent and the supercritical antisolvent, i.e., the solubility of the liquid solvent in the supercritical CO
2 and the quick gas-like diffusion of the scCO
2 in the solvent [
22,
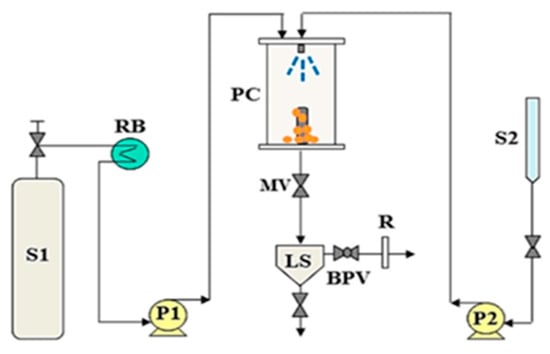
29], as shown in
Figure 3. Several organic liquids that are completely miscible with scCO
2 under process conditions have been used, such as acetic acid, acetone, chloroform, dichloromethane, dimethyl formamide, dimethylsulfoxide, ethanol, ethyl acetate, formic acid, isopropanol, methanol, N-methyl pyrrolidone, and tetrahydrofuran. In some other cases, mixtures of two of the indicated solvents have also been used [
25,
34].
Figure 3. Schematic diagram of the supercritical antisolvent (SAS) apparatus, composed of: BPV: Back-pressure valve; LS: Liquid separator; MV: Micrometric valve; P1, P2: Pumps; PC: precipitation chamber; RB: Refrigeration bath; S1: CO
2 supply; S2: Liquid solution supply; R: Rotameter [
22].