Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Salil Desai | -- | 2539 | 2022-12-08 22:53:04 | | | |
| 2 | Conner Chen | + 54 word(s) | 2593 | 2022-12-12 10:12:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Olowe, M.; Parupelli, S.K.; Desai, S. 3D Printing and Classification of Microneedles. Encyclopedia. Available online: https://encyclopedia.pub/entry/38371 (accessed on 07 February 2026).
Olowe M, Parupelli SK, Desai S. 3D Printing and Classification of Microneedles. Encyclopedia. Available at: https://encyclopedia.pub/entry/38371. Accessed February 07, 2026.
Olowe, Michael, Santosh Kumar Parupelli, Salil Desai. "3D Printing and Classification of Microneedles" Encyclopedia, https://encyclopedia.pub/entry/38371 (accessed February 07, 2026).
Olowe, M., Parupelli, S.K., & Desai, S. (2022, December 08). 3D Printing and Classification of Microneedles. In Encyclopedia. https://encyclopedia.pub/entry/38371
Olowe, Michael, et al. "3D Printing and Classification of Microneedles." Encyclopedia. Web. 08 December, 2022.
Copy Citation
Microneedles are micron-sized devices that are used for the transdermal administration of a wide range of active pharmaceutics substances with minimally invasive pain. 3D-printing technologies that have the potential to revolutionize the manufacturing of microneedles. 3D-printed microneedles have applications in various fields, such as drug delivery, vaccine delivery, cosmetics, therapy, tissue engineering, and diagnostic devices. Microneedles are classified into five types, which include solid microneedles, hollow microneedles, coated microneedles, hydrogel-forming microneedles, and dissolving microneedles.
drug delivery
FDA regulations
microneedles
artificial intelligence
3D printing
1. Introduction
Additive manufacturing (AM) is a technology that is evolving very rapidly and currently has applications in advanced manufacturing and in our day-to-day lives [1][2][3][4][5][6]. It is also referred to as three-dimensional (3D)-printing, layered manufacturing, rapid prototyping, or solid free-form fabrication. This manufacturing approach uses computer-aided design (CAD) files to build three-dimensional objects for applications in the biomedical, health care, manufacturing, fashion, food industry, military, automotive, and aerospace sectors. AM was first developed in the 1980s, when Chuck Hull invented the first form of 3D-printer technology called stereolithography. He was the first to build an AM method that utilized CAD files in order to build 3D objects using rapid prototyping systems. AM technologies build objects from the bottom up by adding material one cross-sectional layer at a time [7][8]. The layers are built in the X-Y direction and are consolidated in order to generate the third dimension, which is the z-dimension. AM gives engineers the flexibility to collaborate and design customizable, complex products from any location in a timely fashion, which in turn breaks the barriers of localized engineering or manufacturing [4][9][10][11][12]. Additive manufacturing mainly consists of the following five basic steps to build 3D objects:
- A computerized 3D solid model is developed;
- It is converted into a standard AM file format, such as the standard tessellation language format (STL) [13];
- The STL file is sent to a 3D printer where it is modified, e.g., changing the position and orientation of the part or scaling the part;
- The part is built layer-by-layer on the 3D-printing machine;
- The cleaning, finishing, and post-processing of the printed parts are conducted.
The advantages and disadvantages of AM processes are illustrated in Table 1. AM processes build three-dimensional objects in a layer-by-layer fashion, as discussed earlier, and can be utilized to rapidly develop 3D structures with complicated designs that are based on a computer-aided design (CAD) model. AM processes are compatible with various types of materials, such as metals, polymers, biomaterials, ceramics, and composites [14][15][16][17][18][19]. The capability to utilize biomaterials in AM processes enables the fabrication of a wide range of 3D structures for clinical and point-of-care applications, including tissue engineering, stem cell research, wound healing, organ-on-chip technology, cancer research assays, and cosmetics [20][21][22][23][24][25].
Table 1. Advantages and disadvantages of additive manufacturing processes.
| Advantages | Disadvantages |
|---|---|
| Designs can be easily changed, and a range of simple to complex structures can be manufactured. | High-resolution printers are expensive and require huge start-up capital. |
| Printing parts can be easily optimized—lightweight and heavy objects can be fabricated. | Build speeds can be slow and high-volume production is limited. |
| Compared to machining, less wastage is generated. | Additional costs might be incurred for the post-processing of finished products for high-quality surface finish. |
| Parts can be manufactured end-to-end without the need for specialized tools. | 3D printers are materials-specific, and this can limit versatility. |
| Complex geometrical structures can be fabricated without any restrictions. |
AM manufacturing processes enable the fabrication of macro- and micro-scale 3D structures for patients with special requirements and materials. AM is a promising technique that can be used for the fabrication of customizable, complex, and cost-effective microneedle arrays. AM techniques such as stereolithography (SLA), selective laser sintering (SLS), digital light processing (DLP), fused deposition modeling (FDM), two-photon polymerization (2PP), and continuous liquid interface production (CLIP) can manufacture microneedle arrays [26][27][28][29]. Generally, the microelectromechanical fabrication techniques such as molding, chemical wet etching, dry etching, direct laser micromachining, ultraviolet (UV) lithography, and micro milling are used for the fabrication of microneedle-based devices. The limitations of the traditional manufacturing techniques include advanced manufacturing facilities, time-consuming processes, expensive specialized equipment, limited customizability, and a lack of flexibility over specific parameters, such as array size, height, and aspect ratio [30]. These above-mentioned limitations can be addressed with the AM processes. Each AM process has a particular set of tradeoffs in terms of biocompatibility, design structure, resolution, cost-effectiveness, material type, and particular application. Other 3D-printing technologies include direct-write inkjet methods that use a combination of different materials and inks [31][32][33][34][35][36][37][38]. The emerging applications of 3D-printed microneedles include healthcare systems, tissue engineering, biomedical engineering, and healthcare systems. These applications specifically include drug and vaccine delivery, cosmetics, therapy, diagnosis, sample extraction, and stem cell research.
2. Microneedles
Microneedles (MNs) are minimally invasive, tiny needle devices that can be fabricated from a variety of materials, such as biomaterials, metals, polymers, ceramics, and composites [39][40][41], which are designed to penetrate the skin’s stratum corneum layer for various applications. The aim of microneedles is the delivery of bioactive materials, vaccines, and pharmaceutical agents, and the collection of bio-signals and substances from the body with minimal invasiveness. The administration of drugs through the gastrointestinal passage has not been the most efficient due to the poor absorption of orally ingested drugs and the pharmacokinetic activities of the body, which leaves only a fraction of the drug to achieve its intended therapeutic effect. Patients’ compliance with the conventional use of hypodermic needles has dropped significantly over the years due to the pain, anxiety, and discomfort that accompany their usage. A more appealing approach that offers the possibility of controlled release at the expense of the time of administration is transdermal drug delivery (TDD) using a microneedle patch. However, TDD is severely limited by the inability of most single drug particles to cross the skin at therapeutic rates due to the great barrier that is imposed by the skin’s stratum corneum layer [42]. In order to increase the skin’s permeability, different approaches have been investigated, including, but not limited to, chemical lipid enhancers, electric field employing iontophoresis, and electroporation to pressure waves that are generated by ultrasound or photoacoustic effects [43]. An alternative approach involves creating a pathway of micron-scale needles that serves to create microscopic holes on the outermost layer, called the stratum corneum, by inserting microneedles that are made of silicon, metal, or polymeric material. Microneedle arrays are promising devices in transdermal drug delivery applications.
Microneedles are designed to be able to create an easier passage to the rich blood supply in the lower dermal layers, allowing an easy, pain-free delivery of a wide range of medicines across the skin [44]. The advantages of microneedles include painless administration, faster healing, ease of administration, and more control over the rate of drug delivery. Microneedle patches are categorized into five types, as shown in Figure 1, which include the following: solid microneedles, coated microneedles, dissolvable microneedles, hollow microneedles, and hydrogel-based microneedles. Each microneedle type has particular fabrication procedures and application areas. The first report of the term “microneedle” dates back to 1921 (Chambers, 1921), as a means of the micro-dissection of echinoderm eggs [45].
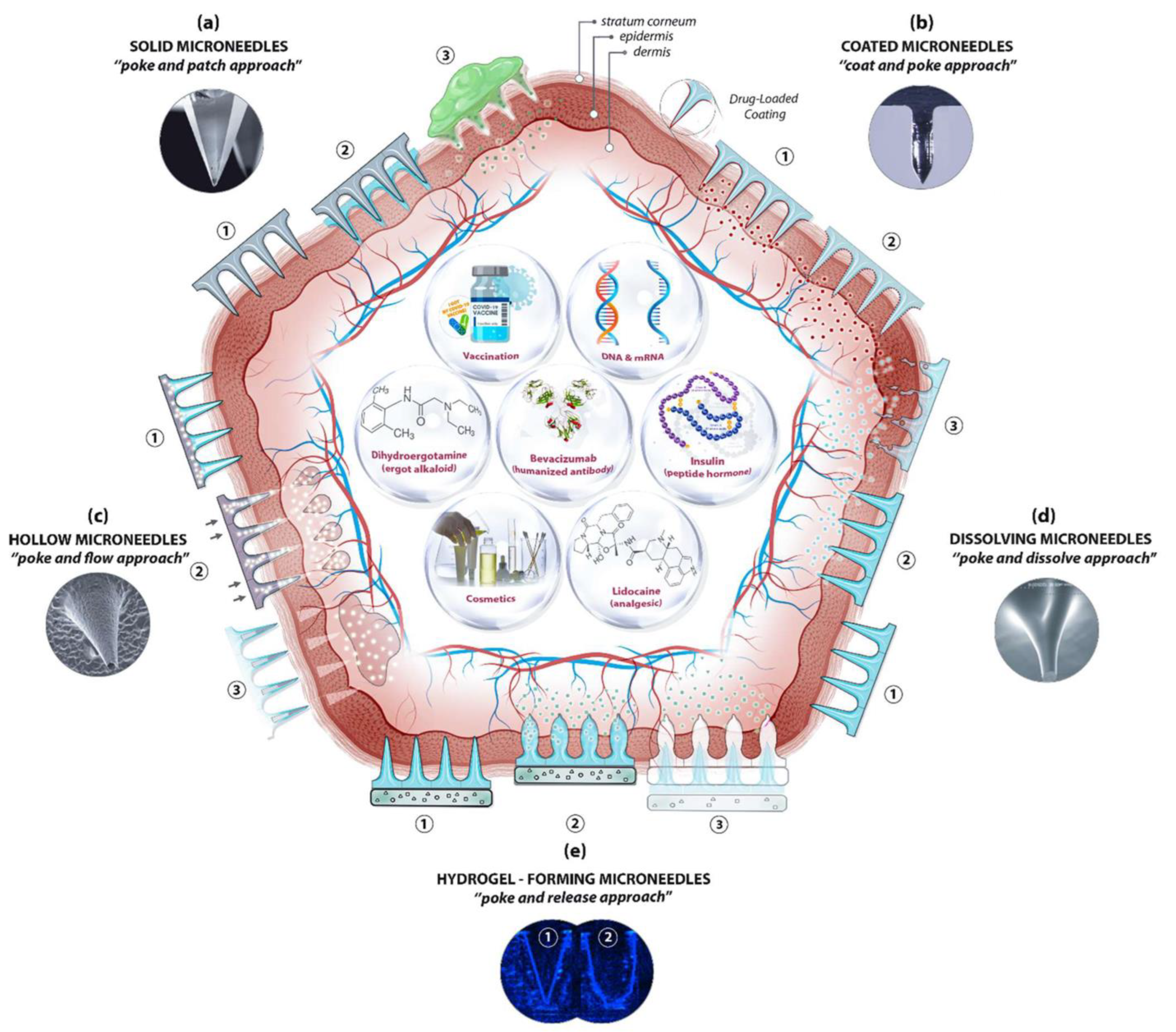
Figure 1. A schematic diagram of microneedle-based drug delivery approaches with a cross-section of the upper layer of the skin. The approaches are (a) solid microneedles, (b) coated microneedles, (c) hollow microneedles, (d) dissolving microneedles, and (e) hydrogel-forming microneedles. The step-by-step process of each delivery approach is numbered from 1 to 3 [46].
The microneedle concept started with using large needles, until it evolved over the years to the current micro-sized needles. In 1905, a German dermatologist used a motor-powered dental brush to treat skin ailments [47]. In the 1970s, Gerstel et al. introduced the microneedle concept, however, this concept was not demonstrated experimentally until the 1990s [48]. In 1998, Henry et al. were the first to propose a microneedle to be used for transdermal drug delivery [49]. The microneedle array in their study was fabricated using silicon as the material, with etching and photolithography as the manufacturing techniques. Initially, the purpose of the microneedle was to increase the skin’s permeability by using a solid microneedle. Another purpose was to fabricate hollow microneedles with advanced functionality compared to the ordinary hypodermic needles [50]. Eventually, this concept was extended to different applications, such as drug delivery, vaccine delivery, therapeutics, diagnostics, and cosmetic applications. A variety of materials, such as silicon, stainless steel, sugar, and polymers, have been used in order to fabricate solid microneedles, coated microneedles, hollow microneedles, or dissolvable microneedles [51]. Silicon was the first proposed material for fabricating a solid microneedle [49]; however, many other materials were studied in order to manufacture microneedles, such as stainless steel [52], ceramic [53], glass [54], and polymer [55]. Various types of manufacturing methods and techniques have been utilized over the years to fabricate specific and customized microneedle arrays. These manufacturing methods include, but are not limited to, lithography [56][57], micro milling, mold-based techniques, injection molding [58], laser ablation [59][60], an elasto-capillarity-driven self-assembly mechanism, and additive manufacturing [27][61]. Many of these conventional fabrication methods have some limitations, such as cost-efficiency, requiring manual steps, requiring sophisticated equipment, and being labor-intensive. Hence, accessible and cost-efficient technologies, such as additive manufacturing, are needed in order to produce microneedles. A summary of the fabrication techniques for the different types of microneedles is illustrated in Table 2.
Table 2. Source: “Microneedles: A smart approach and increasing potential for transdermal drug delivery system” [62].
| S/N | MICRONEEDLE TYPE | Fabrication Techniques |
|---|---|---|
| 1. | Solid Microneedles | |
| Metal microneedles |
|
|
| Silicon microneedles |
|
|
| Polymer microneedles |
|
|
| Ceramic microneedles |
|
|
| 2. | Hollow microneedles |
|
| 3. | Coated microneedles |
|
| 4. | Dissolvable microneedles |
|
| 5. | Hydrogel-forming microneedles |
|
3. Classification of Microneedles
As mentioned earlier, microneedles are classified into five types, as shown in Figure 2, which include solid microneedles, hollow microneedles, coated microneedles, hydrogel-forming microneedles, and dissolving microneedles. The details about the material composition of microneedles and their benefits were summarized in Table 3.
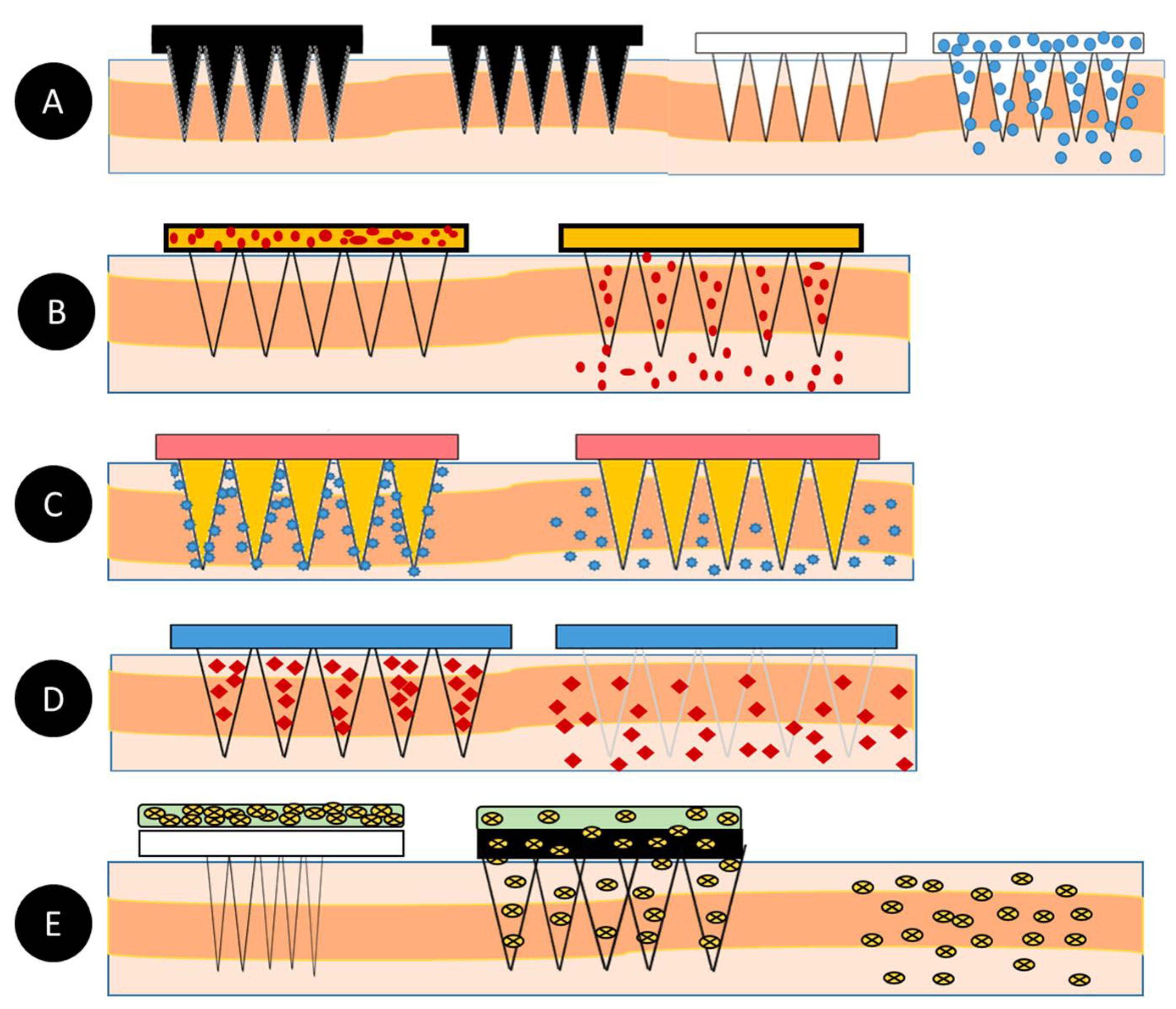
Figure 2. (A)—Solid microneedles; (B)—Hollow microneedles; (C)—Coated microneedles; (D)—Dissolving microneedles; (E)—Hydrogel-forming microneedles [40].
Table 3. An overview of the different types of microneedle material characteristics and benefits [51].
| S/N | Microneedle Type | Characteristics | Advantages | Disadvantages | Applications | Material |
|---|---|---|---|---|---|---|
| 1 | Solid | Creates holes in the skin for easy delivery of drugs to the lower layers of the skin | Can be easily fabricated | Prone to infection | Drug delivery and Cosmetics | Silicon Polymer Metal |
| 2 | Hollow | The drug is filled into the empty space for controlled delivery | Able to handle a large volume of drug solution | Leakage and clogging could occur, and the needle design and insertion methods could pose a challenge | Diagnosis of disease | Silicon |
| 3 | Coated | Based on its design, it carries a lower amount of drugs | Quick delivery of drugs to the skin | Susceptible to infections | Drug and Vaccine Delivery | Silicon |
| 4 | Dissolving | Rapid release of macromolecules | Can be easily administered to patients with a one-set application | Takes some time to dissolve, and requires expertise to manufacture | Drug delivery Vaccine delivery Cosmetics |
Silicon |
| 5 | Hydrogel-forming | Absorbs fluids (due to its hydrophilic nature) and swells, creating channels for the delivery of drug molecules | Can be applied to the skin leaving no residues, and has no clogging, unlike hollow microneedles | Potential for localized tissue damage, and has a slow swelling rate | ISF extraction Transdermal drug delivery Disease treatment |
Polymer |
3.1. Solid Microneedles
Solid microneedles can be used to create microscopic holes in the skin through which molecules and therapeutic agents can be easily transported. This type of microneedle structure is designed to penetrate the stratum corneum in order to enhance the drug delivery to the dermis in order to improve the bioavailability and the kinetic transport across the skin [51]. The first microneedle arrays that were reported in the literature were etched into a silicon wafer and were developed for intracellular delivery in vitro by Hashmi et al. [63]. In comparison with hollow microneedles, solid microneedles have better mechanical properties [64][65], and the sharper tips are easier to manufacture. Solid microneedles are mostly used for pre-treating the skin by forming pores [66][67][68]. The pointed tips of the needles penetrate the skin, creating channels of micron size through which the drug directly enters the skin layers via the application of a drug patch, thus increasing the permeation [62].
3.2. Hollow Microneedles
Hollow microneedles can accommodate a large dose of the drug dispersion or solution, as higher amounts can fill up the empty space inside of the needle. They are mostly used for high molecular weight compounds, such as proteins and vaccines [69]. Unlike solid microneedles, hollow microneedles are active drug delivery systems that form a channel for the efficient diffusion of drugs into the dermal layer based on a non-pressurized drug reservoir [51]. Hollow microneedles can be designed to allow for the modulation of the flow, the pressure, and the drug release rate [51][70]. The microneedle aspect ratio can be controlled for a rapid release, a slow infusion, or a time-varying delivery rate [71]. Mishra et al. developed hollow microneedles that were aligned on the silicon substrate with a length of 500–600 μm and a 100 μm outer diameter that achieved a flow rate of 0.93 μL s−1 at a pressure differential of 2 KPa [72][73]. Hollow microneedles have the disadvantage of clogging and leakage during the injection procedure. They are also relatively weaker and require intensive care in terms of the needle design and the insertion methods [74].
3.3. Coated Microneedles
A coated microneedle comprises a sharp, solid-core microneedle structure on which a solid film containing the active compound and water-soluble inactive excipients are coated [75]. A coated microneedle can deliver proteins and DNA in a minimally invasive manner [76]. An advantage of a coated microneedle is the rapid delivery of the drug to the skin; however, the remnant drug at the tip of the needle might infect other patients [76]. In contrast to the dissolvable microneedle, whose mechanical properties can change when the encapsulated drug fraction is altered or when a different drug is dispersed in its matrix, the mechanical properties of a solid microneedle are not impacted when a different drug is coated on its surface [75].
3.4. Dissolving Microneedles
Dissolving microneedles are made from biodegradable polymers [77][78]. They are designed to encapsulate the drug agents and to control their release upon the degradation and dissolution of the polymers into the skin. This type of microneedle differs from the others in that it does not have to be removed after its insertion. The bio-acceptability and the dissolution of the polymer inside the skin make it one of the best choices for long-term therapy, with improved patient compliance [79]. Water-soluble materials are the most suitable for manufacturing dissolvable microneedles. In addition, the micro-molding method is one of the most appropriate techniques for producing dissolvable microneedles. The micro-molding procedure involves filling the microneedle mold with a concentrated polymer solution and drying or filling it with melted polymer and allowing it to solidify. One of the biggest challenges of using dissolvable microneedles is that there can be a delay in the dissolution and complete insertion is impracticable [62].
3.5. Hydrogel-Forming Microneedles
Hydrogel-forming microneedles are usually made up of swelling materials or aqueous polymer gels. This type of microneedle works by absorbing the interstitial fluid when it is applied to the skin and swells, resulting in the formation of channels between the capillary circulation and the drug patch. Upon the application and the swelling of these microneedles, they behave as a rate-controlling membrane. Hydrogel-forming microneedles can be easily sterilized and removed from the skin where they are applied [79][80][81]. Hydrogel-forming microneedles can also be fabricated using cross-linked polymers. Hydrogel-forming microneedles help to improve the permeation and bioavailability when they are used in transdermal drug delivery [50][82].
References
- Aldawood, F.K.; Desai, S.; Chang, S. Additive Manufacturing of Compensator Devices for Radiation Therapy. In Proceedings of the 2020 IISE Annual Conference, New Orleans, LA, USA, 30 May–2 June 2020.
- Parupelli, S.K.; Desai, S. Understanding Hybrid Additive Manufacturing of Functional Devices. Am. J. Eng. Appl. Sci. 2017, 10, 264–271.
- Aljohani, A.; Desai, S. 3D Printing of Porous Scaffolds for Medical Applications. Am. J. Eng. Appl. Sci. 2018, 11, 1076–1085.
- Haeberle, G.; Desai, S. Investigating Rapid Thermoform Tooling Via Additive Manufacturing (3d Printing). Am. J. Appl. Sci. 2019, 16, 238–243.
- Desai, S.; Parupelli, S. Additive Manufacturing (3D Printing). In Maynard’s Industrial and Systems Engineering Handbook, 6th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; ISBN 1260461564.
- Adarkwa, E.; Kotoka, R.; Desai, S. 3D printing of polymeric Coatings on AZ31 Mg alloy Substrate for Corrosion Protection of biomedical implants. Med. Devices Sens. 2021, 4, e10167.
- Slotwinski, J.A.; Campbell, T.A. Metrology for Additive Manufacturing—Opportunities in a Rapidly Emerging Technology; NOVA Science Publishers: Hauppauge, NY, USA, 2013; Volume 7, pp. 153–174.
- Ivanova, O.; Williams, C.; Campbell, T. Additive manufacturing (AM) and nanotechnology: Promises and challenges. Rapid Prototyp. J. 2013, 19, 353–364.
- McKenzie, J.; Desai, S. Investigating Sintering Mechanisms for Additive Manufacturing of Conductive Traces. Am. J. Eng. Appl. Sci. 2018, 11, 652–662.
- Parupelli, S.K.; Desai, S. Hybrid additive manufacturing (3D printing) and characterization of functionally gradient materials via in situ laser curing. Int. J. Adv. Manuf. Technol. 2020, 110, 543–556.
- Adarkwa, E.; Roy, A.; Ohodnicki, J.; Lee, B.; Roy, A.; Kumta, P.; Desai, S. 3D printing of drug-eluting bioactive multifunctional coatings for orthopedic applications. Int. J. Bioprinting 2023, 9, 0119.
- Aldawood, F.K.; Chang, S.X.; Desai, S. Design and Manufacture of a High Precision Personalized Electron Bolus Device for Radiation Therapy. Med. Devices Sens. 2020, 3, e10077.
- Kumar, V.; Dutta, D. An assessment of data formats for layered manufacturing. Adv. Eng. Softw. 1997, 28, 151–164.
- Desai, S.; Bidanda, B.; Bártolo, P. Metallic and ceramic biomaterials: Current and future developments. In Bio-Materials and Prototyping Applications in Medicine; Springer: Boston, MA, USA, 2008; pp. 1–14.
- Desai, S.; Shankar, M.R. Chapter 2 Polymers, Composites and Nano Biomaterials: Current and Future Developments. In Bio-Materials and Prototyping Applications in Medicine; Springer: Boston, MA, USA, 2008; pp. 15–26.
- Desai, S.; Harrison, B. Direct-Writing of Biomedia for Drug Delivery and Tissue Regeneration. Printed Biomaterials; Springer: New York, NY, USA, 2010; pp. 71–89.
- Perkins, J.; Xu, Z.; Smith, C.; Roy, A.; Kumta, P.N.; Waterman, J.; Conklin, D.; Desai, S. Direct Writing of Polymeric Coatings on Magnesium Alloy for Tracheal Stent Applications. Ann. Biomed. Eng. 2015, 43, 1158–1165.
- Desai, S.; Bidanda, B.; Bártolo, P.J. Emerging Trends in the Applications of Metallic and Ceramic Biomaterials. Bio-Materials and Prototyping Applications in Medicine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–17.
- Desai, S.; Shankar, M.R. Emerging Trends in Polymers, Composites and Nano Biomaterial Applications. Bio-Materials & Prototyping Applications in Medicine; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-030-35875-4.
- Marquetti, I.D.S. Analyzing Bone Morphogenetic Protein-2 Adsorption Behavior on Hydrophobic Graphite Substrate. In Proceedings of the Brazilian Graduate Students and Scholars Conference (BRASCON), Columbus, OH, USA, 23–24 June 2018.
- Haeberle, G.; Desai, S. Additive Manufacturing (3D Printing) of Thermoform Tooling. Int. J. Mech. Prod. Eng. 2019, 7, 1–4.
- Perkins, J.; Yi, H.; Ye, S.H.; Wagner, W.; Desai, S. Direct Write Manufacturing of Controlled Release Coatings for Drug Eluting Cardiovascular Stents. J. Biomed. Res. Part A 2014, 102, 4290–4300.
- Marquetti, I. Molecular Modeling of Bone Morphogenetic Protein for Tissue Engineering Applications. In Proceedings of the Industrial Engineers Research Conference, Orlando, FL, USA, 19–22 May 2018; Volume 2, pp. 1108–1113.
- Marquetti, I.; Desai, S. Adsorption Behavior of Bone Morphogenetic Protein-2 on a Graphite Substrate for Biomedical Applications. Am. J. Eng. Appl. Sci. 2018, 11, 1037–1044.
- Adarkwa, E.; Desai, S.; Ohodnicki, J.M.; Roy, A.; Lee, B.; Kumta, P.N. Amorphous Calcium Phosphate Blended Polymer Coatings for Biomedical Implants. In Proceedings of the 2014 Industrial and Systems Engineering Research Conference, Montréal, QC, Canada, 31 May–3 June 2014.
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip 2018, 18, 1223–1230.
- Krieger, K.J.; Bertollo, N.; Dangol, M.; Sheridan, J.T.; Lowery, M.M.; O’Cearbhaill, E.D. Simple and customizable method for fabrication of high-aspect ratio microneedle molds using low-cost 3D printing. Microsyst. Nanoeng. 2019, 5, 42.
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2015, 42, 1019–1031.
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483.
- Donnelly, R.F.; Singh TR, R.; Morrow, D.I.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; John Wiley and Sons: Hoboken, NJ, USA, 2012.
- Desai, S.; Lovell, M. Multiphysics Modeling of a Piezoelectric Bimorph Disc in a Direct Write Fabrication Process. In Proceedings of the ASME 2005 International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; Volume 100, pp. 437–442.
- Adarkwa, E.; Desai, S. Scalable Droplet Based Manufacturing Using In-Flight Laser Evaporation. J. Nanoeng. Nanomanufacturing 2016, 6, 87–92.
- Desai, S.; Lovell, M. Modeling fluid–structure interaction in a direct write manufacturing process. J. Mater. Process. Technol. 2012, 212, 2031–2040.
- Cordeiro, J.; Desai, S. Process Parameter Studies of Molecular Dynamics Models to Control Substrate Wettability. In Proceedings of the International Manufacturing Science and Engineering Conference, Guangzhou, China, 28–29 November 2015.
- Desai, S.; Lovell, M. CFD Analysis of a Continuous Inkjet Print Head for Direct Write Fabrication. In Proceedings of the ASME 2007 International Mechanical Engineering Congress and Exposition, Seattle, WC, USA, 11–15 November 2007; Volume 13, pp. 209–213.
- Cordeiro, J.; Desai, S. Exploring Nano Scale Design Space with Molecular Dynamics Simulations. In Proceedings of the 2015 Industrial and Systems Engineering Research Conference, Nashville, TN, USA, 30 May–2 June 2015; p. 856. Available online: https://www.proquest.com/openview/15b4103286e4d16481a08298800d3854/1?pq-origsite=gscholar&cbl=51908 (accessed on 12 October 2022).
- Desai, S. Methods and Apparatus for Manufacturing Micro-and/or Nano-Scale Features. U.S. Patent Application 13/959,849, 28 November 2013.
- Desai, S.; Lovell, M. Coupled field analysis of a piezoelectric bimorph disc within a CIJ microfabrication process. In Proceedings of the 2006 IIE Annual Conference and Exhibition, Orlando, FL, USA, 20–24 May 2006.
- Erdem, Ö.; Eş, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent Advances in Microneedle-Based Sensors for Sampling, Diagnosis and Monitoring of Chronic Diseases. Biosensors 2021, 11, 296.
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717.
- Ali, R.; Mehta, P.; Arshad, M.; Kucuk, I.; Chang, M.W.; Ahmad, Z. Transdermal Microneedles—A Materials Perspective. AAPS PharmSciTech 2020, 21, 1–14.
- Bronaugh, R.L.; Maibach, H.I. (Eds.) Percutaneous Absorption: Drugs, Cosmetics, Mechanisms, Methods; CRC Press: Boca Raton, FL, USA, 2021.
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587.
- Escobar-Chávez, J.J.; Bonilla-Martínez, D.; Villegas-González, M.A.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vázquez, A.L. Microneedles: A Valuable Physical Enhancer to Increase Transdermal Drug Delivery. J. Clin. Pharmacol. 2011, 51, 964–977.
- Dabbagh, S.R.; Sarabi, M.R.; Rahbarghazi, R.; Sokullu, E.; Yetisen, A.K.; Tasoglu, S. 3D-printed microneedles in biomedical applications. iScience 2021, 24, 102012.
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321.
- Walsh, L. Microneedling: A versatile and popular treatment option. J. Aesthetic Nurs. 2019, 8, 280–284.
- Gerstel, M.S.; Place, V.A. Drug Delivery Device. U.S. Patent US3964482A, 22 June 1976.
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925.
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568.
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815.
- Verbaan, F.J.; Bal, S.M.; Van den Berg, D.J.; Groenink, W.H.H.; Verpoorten, H.; Lüttge, R.; Bouwstra, J.A. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245.
- Ovsianikov, A.; Chichkov, B.; Mente, P.; Monteiro-Riviere, N.A.; Doraiswamy, A.; Narayan, R.J. Two Photon Polymerization of Polymer–Ceramic Hybrid Materials for Transdermal Drug Delivery. Int. J. Appl. Ceram. Technol. 2007, 4, 22–29.
- Wang, J.; Lu, J.; Ly, S.Y.; Vuki, M.; Tian, B.; Adeniyi, W.K.; Armendariz, R.A. Lab-on-a-Cable for electrochemical monitoring of phenolic contaminants. Anal. Chem. 2000, 72, 2659–2663.
- Park, J.H.; Allen, M.G.; Prausnitz, M.R. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J. Control. Release 2005, 104, 51–66.
- Ren, L.; Jiang, Q.; Chen, Z.; Chen, K.; Xu, S.; Gao, J.; Jiang, L. Flexible microneedle array electrode using magnetorheological drawing lithography for bio-signal monitoring. Sens. Actuators A Phys. 2017, 268, 38–45.
- Lee, K.; Lee, H.C.; Lee, D.S.; Jung, H. Drawing Lithography: Three-Dimensional Fabrication of an Ultrahigh-Aspect-Ratio Microneedle. Adv. Mater. 2010, 22, 483–486.
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043.
- Al-Muriesh, M.; Huang, C.Z.; Ye, Z.; Yang, J. Dermoscopy and VISIA imager evaluations of non-insulated microneedle radiofrequency versus fractional CO2 laser treatments of striae distensae. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1859–1866.
- Tu, K.T.; Chung, C.K. Rapid prototyping of biodegradable microneedle arrays by integrating CO2 laser processing and polymer molding. J. Micromechanics Microengineering 2016, 26, 065015.
- Johnson, A.R.; Procopio, A.T. Low cost additive manufacturing of microneedle masters. 3D Print. Med. 2019, 5, 2.
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258.
- Hashmi, S.; Hashmi, G.; Gaugler, R. Genetic Transformation of an Entomopathogenic Nematode by Microinjection. J. Invertebr. Pathol. 1995, 66, 293–296.
- Prausnitz, M.R.; Mikszta, J.A.; Raeder-Devens, J. Microneedles. In Percutaneous Penetration Enhancers; CRC Press: Boca Raton, FL, USA, 2005; pp. 253–270.
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and progress. Drug Deliv. 2014, 23, 2338–2354.
- Sabri, A.H.; Ogilvie, J.; Abdulhamid, K.; Shpadaruk, V.; McKenna, J.; Segal, J.; Scurr, D.J.; Marlow, M. Expanding the applications of microneedles in dermatology. Eur. J. Pharm. Biopharm. 2019, 140, 121–140.
- Tariq, N.; Ashraf, M.W.; Tayyaba, S. A Review on Solid Microneedles for Biomedical Applications. J. Pharm. Innov. 2021, 1–20.
- Mishra, P.; Gautam, V.; Sharma, R.K.; Tiwari, A.; Sawarkar, A. Microneedle: A useful tool for drug delivery system. J. Pharm. Phytochem. 2020, 9, 340–345.
- Suh, H.; Shin, J.; Kim, Y.-C. Microneedle patches for vaccine delivery. Clin. Exp. Vaccine Res. 2013, 3, 42–49.
- Mahato, R. Chapter 13—Microneedles in Drug Delivery. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–353.
- Mishra, R.; Maiti, T.K.; Bhattacharyya, T.K. Design and scalable fabrication of hollow SU-8 microneedles for transdermal drug delivery. IEEE Sens. J. 2018, 18, 5635–5644.
- van der Maaden, K.; Jiskoot, W.; Bouwstra, J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J. Control. Release 2012, 161, 645–655.
- Zhang, P.; Jullien, G.A. Microneedle arrays for drug delivery and fluid extraction. In Proceedings of the 2005 International Conference on MEMS, NANO and Smart Systems, ICMENS 2005, Banff, AB, Canada, 24–27 July 2005; pp. 392–395.
- Zhang, P.; Dalton, C.; Jullien, G.A. Design and fabrication of MEMS-based microneedle arrays for medical applications. Microsyst. Technol. 2009, 15, 1073–1082.
- Ingrole, R.S.J.; Gill, H.S. Microneedle Coating Methods: A Review with a Perspective. J. Pharmacol. Exp. Ther. 2019, 370, 555–569.
- Duong, H.T.T.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Phan, V.H.G.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 2018, 185, 13–24.
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A review of recent advances in microneedle technology for transdermal drug delivery. J. Drug. Deliv. Sci. Technol. 2020, 59, 101923.
- Tomono, T. A new way to control the internal structure of microneedles: A case of chitosan lactate. Mater. Today Chem. 2019, 13, 79–87.
- Ita, K. Transdermal Delivery of Drugs with Microneedles—Potential and Challenges. Pharmaceutics 2015, 7, 90–105.
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890.
- Hong, X.; Wu, Z.; Chen, L.; Wu, F.; Wei, L.; Yuan, W. Hydrogel Microneedle Arrays for Transdermal Drug Delivery. Nano-Micro Lett. 2014, 6, 191–199.
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.A.; McCrudden, M.T.C.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.E. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.3K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




