Microneedles are micron-sized devices that are used for the transdermal administration of a wide range of active pharmaceutics substances with minimally invasive pain. 3D-printing technologies that have the potential to revolutionize the manufacturing of microneedles. 3D-printed microneedles have applications in various fields, such as drug delivery, vaccine delivery, cosmetics, therapy, tissue engineering, and diagnostic devices. Microneedles are classified into five types, which include solid microneedles, hollow microneedles, coated microneedles, hydrogel-forming microneedles, and dissolving microneedles. This review enumerates the challenges that are posed by the 3D-printing technologies, including the manufacturing cost, which limits its viability for large-scale production, the compatibility of the microneedle-based materials with human cells, and concerns around the efficient administration of large dosages of loaded microneedles.
- drug delivery
- FDA regulations
- microneedles
- artificial intelligence
- 3D printing
1. Introduction
- A computerized 3D solid model is developed;
- It is converted into a standard AM file format, such as the standard tessellation language format (STL) [13];
- The STL file is sent to a 3D printer where it is modified, e.g., changing the position and orientation of the part or scaling the part;
- The part is built layer-by-layer on the 3D-printing machine;
- The cleaning, finishing, and post-processing of the printed parts are conducted.
-
A computerized 3D solid model is developed;
-
It is converted into a standard AM file format, such as the standard tessellation language format (STL) [13];
-
The STL file is sent to a 3D printer where it is modified, e.g., changing the position and orientation of the part or scaling the part;
-
The part is built layer-by-layer on the 3D-printing machine;
-
The cleaning, finishing, and post-processing of the printed parts are conducted.
| Advantages | Disadvantages |
|---|---|
| Designs can be easily changed, and a range of simple to complex structures can be manufactured. | High-resolution printers are expensive and require huge start-up capital. |
| Printing parts can be easily optimized—lightweight and heavy objects can be fabricated. | Build speeds can be slow and high-volume production is limited. |
| Compared to machining, less wastage is generated. | Additional costs might be incurred for the post-processing of finished products for high-quality surface finish. |
| Parts can be manufactured end-to-end without the need for specialized tools. | 3D printers are materials-specific, and this can limit versatility. |
| Complex geometrical structures can be fabricated without any restrictions. |
2. Microneedles
Microneedles (MNs) are minimally invasive, tiny needle devices that can be fabricated from a variety of materials, such as biomaterials, metals, polymers, ceramics, and composites [39][40][41][39,40,41], which are designed to penetrate the skin’s stratum corneum layer for various applications. The aim of microneedles is the delivery of bioactive materials, vaccines, and pharmaceutical agents, and the collection of bio-signals and substances from the body with minimal invasiveness. The administration of drugs through the gastrointestinal passage has not been the most efficient due to the poor absorption of orally ingested drugs and the pharmacokinetic activities of the body, which leaves only a fraction of the drug to achieve its intended therapeutic effect. Patients’ compliance with the conventional use of hypodermic needles has dropped significantly over the years due to the pain, anxiety, and discomfort that accompany their usage. A more appealing approach that offers the possibility of controlled release at the expense of the time of administration is transdermal drug delivery (TDD) using a microneedle patch. However, TDD is severely limited by the inability of most single drug particles to cross the skin at therapeutic rates due to the great barrier that is imposed by the skin’s stratum corneum layer [42]. In order to increase the skin’s permeability, different approaches have been investigated, including, but not limited to, chemical lipid enhancers, electric field employing iontophoresis, and electroporation to pressure waves that are generated by ultrasound or photoacoustic effects [43]. An alternative approach involves creating a pathway of micron-scale needles that serves to create microscopic holes on the outermost layer, called the stratum corneum, by inserting microneedles that are made of silicon, metal, or polymeric material. Microneedle arrays are promising devices in transdermal drug delivery applications. Microneedles are designed to be able to create an easier passage to the rich blood supply in the lower dermal layers, allowing an easy, pain-free delivery of a wide range of medicines across the skin [44]. The advantages of microneedles include painless administration, faster healing, ease of administration, and more control over the rate of drug delivery. Microneedle patches are categorized into five types, as shown in Figure 1, which include the following: solid microneedles, coated microneedles, dissolvable microneedles, hollow microneedles, and hydrogel-based microneedles. Each microneedle type has particular fabrication procedures and application areas. The first report of the term “microneedle” dates back to 1921 (Chambers, 1921), as a means of the micro-dissection of echinoderm eggs [45].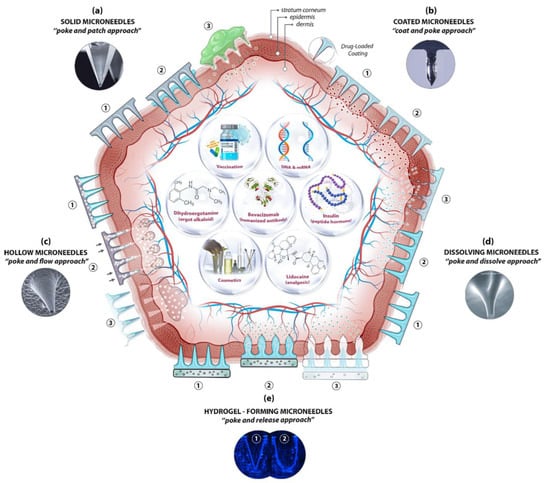
| S/N | MICRONEEDLE TYPE | Fabrication Techniques |
|---|---|---|
| 1. | Solid Microneedles | |
| Metal microneedles |
| |
| ||

3. Classification of Microneedles
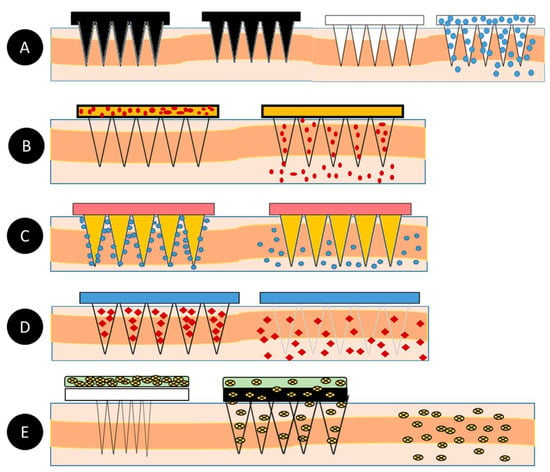
| S/N | Microneedle Type | Characteristics | Advantages | Disadvantages | Applications | Material | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Solid | Creates holes in the skin for easy delivery of drugs to the lower layers of the skin | Can be easily fabricated | Prone to infection | Drug delivery and Cosmetics | Silicon Polymer Metal |
|||
|
|
||||||||
| 2 | Hollow | The drug is filled into the empty space for controlled delivery | Able to handle a large volume of drug solution | Leakage and clogging could occur, and the needle design and insertion methods could pose a challenge | Diagnosis of disease | Silicon | Silicon microneedles | ||
| 3 |
|
Coated |
|
||||||
| Based on its design, it carries a lower amount of drugs | Quick delivery of drugs to the skin | Susceptible to infections | Drug and Vaccine Delivery | Silicon | Polymer microneedles |
|
|||
| 4 | Dissolving | Rapid release of macromolecules | Can be easily administered to patients with a one-set application | Takes some time to dissolve, and requires expertise to manufacture | Drug delivery Vaccine delivery Cosmetics |
Silicon | Ceramic microneedles |
|
|
| 5 | Hydrogel-forming | Absorbs fluids (due to its hydrophilic nature) and swells, creating channels for the delivery of drug molecules | Can be applied to the skin leaving no residues, and has no clogging, unlike hollow microneedles | Potential for localized tissue damage, and has a slow swelling rate | ISF extraction Transdermal drug delivery Disease treatment |
Polymer | 2. | Hollow microneedles |
|
| 3. | Coated microneedles |
|
|||||||
| 4. | Dissolvable microneedles |
|
|||||||
| 5. | Hydrogel-forming microneedles |
|
