
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ciro Romano | -- | 2565 | 2022-12-01 10:25:27 | | | |
| 2 | Vivi Li | -37 word(s) | 2528 | 2022-12-02 03:58:34 | | |
Video Upload Options
Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic disorder characterized by peripheral eosinophilia, severe eosinophilic asthma, sinusitis, transient pulmonary infiltrates, and features of medium/small-vessel vasculitis. EGPA belongs to the group of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitides, although only 30 to 40% of patients display ANCA positivity, which is mainly of myeloperoxidase (MPO) specificity. Particularly, ANCA-positive patients typically show vasculitic features. Interleukin (IL)-5 has been demonstrated to play a crucial role in determining eosinophilic airway inflammation in EGPA patients. Specifically, maturation, activation, and survival of eosinophils especially depend on IL-5 availability. Therefore, blocking IL-5 biological activity may be a rewarding strategy for control of eosinophilic inflammation. Several monoclonal antibodies with the ability to interfere with the biological activity of IL-5 have been developed, namely, mepolizumab, reslizumab, and benralizumab.
1. Introduction
2. Therapeutic Management of EGPA
2.1. Standard Therapy
| Original 1996 FFS | Revised 2011 FFS |
|---|---|
| Cardiac involvement Gastrointestinal disease (bleeding, perforation, infarction, or pancreatitis) Renal insufficiency (plasma creatinine concentration >1.6 mg/dL [141 mmol/L]) Proteinuria (>1 g/day) Central nervous system involvement |
Age > 65 years Cardiac insufficiency Renal insufficiency (stabilized peak creatinine 1.7 mg/dL [150 micromol/L]) Gastrointestinal involvement Absence of ENT manifestations (presence is associated with a better prognosis) |
2.2. Anti-IL-5-Targeted Therapies
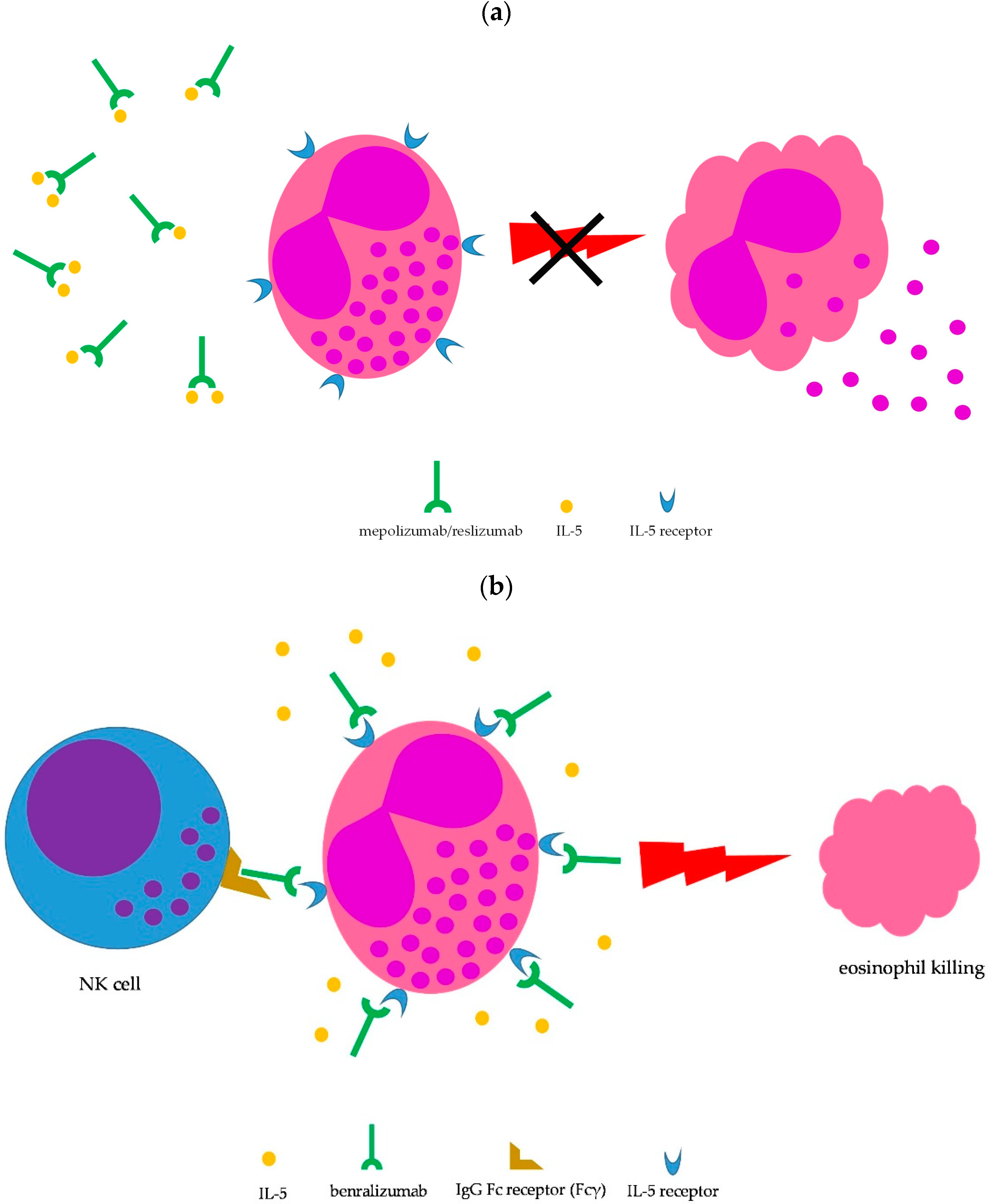
2.2.1. Mepolizumab
2.2.2. Reslizumab
2.2.3. Benralizumab
References
- Almaani, S.; Fussner, L.A.; Brodsky, S.; Meara, A.S.; Jayne, D. ANCA-Associated Vasculitis: An Update. J. Clin. Med. 2021, 10, 1446.
- Kitching, A.R.; Anders, H.J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 2020, 6, 71.
- Radice, A.; Bianchi, L.; Sinico, R.A. Anti-neutrophil cytoplasmic autoantibodies: Methodological aspects and clinical significance in systemic vasculitis. Autoimmun. Rev. 2013, 12, 487–495.
- Cornec, D.; Cornec-Le Gall, E.; Fervenza, F.C.; Specks, U. ANCA-associated vasculitis—Clinical utility of using ANCA specificity to classify patients. Nat. Rev. Rheumatol. 2016, 12, 570–579.
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11.
- Prete, M.; Indiveri, F.; Perosa, F. Vasculitides: Proposal for an integrated nomenclature. Autoimmun. Rev. 2016, 15, 167–173.
- Koike, H.; Nishi, R.; Yagi, S.; Furukawa, S.; Fukami, Y.; Iijima, M.; Katsuno, M. A Review of Anti-IL-5 Therapies for Eosinophilic Granulomatosis with Polyangiitis. Adv. Ther. 2022. online ahead of print.
- Comarmond, C.; Pagnoux, C.; Khellaf, M.; Cordier, J.F.; Hamidou, M.; Viallard, J.F.; Maurier, F.; Jouneau, S.; Bienvenu, B.; Puéchal, X.; et al. French Vasculitis Study Group. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term followup of the 383 patients enrolled in the French vasculitis study group cohort. Arthritis Rheum. 2013, 65, 270–281.
- Chaigne, B.; Guillevin, L. Vasculitis for the internist: Focus on ANCA-associated vasculitis. Intern. Emerg. Med. 2017, 12, 577–585.
- Zarka, F.; Veillette, C.; Makhzoum, J.P. A Review of Primary Vasculitis Mimickers Based on the Chapel Hill Consensus Classification. Int. J. Rheumatol. 2020, 2020, 8392542.
- Grayson, P.C.; Ponte, C.; Suppiah, R.; Robson, J.C.; Craven, A.; Judge, A.; Khalid, S.; Hutchings, A.; Luqmani, R.A.; Watts, R.A.; et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology Classification Criteria for Eosinophilic Granulomatosis with Polyangiitis. Ann. Rheum. Dis. 2022, 81, 309–314.
- Chung, S.A.; Langford, C.A.; Maz, M.; Abril, A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Care Res. 2021, 73, 1088–1105.
- Guillevin, L.; Lhote, F.; Gayraud, M.; Cohen, P.; Jarrousse, B.; Lortholary, O.; Thibult, N.; Casassus, P. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine 1996, 75, 17–28.
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P.L.; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27.
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939.
- Diagnosis and Management of Difficult-to-Treat & Severe Asthma—Global Initiative for Asthma—GINA (ginasthma.org). Available online: https://ginasthma.org/severeasthma/ (accessed on 23 October 2022).
- Desai, M.; Oppenheimer, J. Biologics in allergic and immunologic diseases: Promises and challenges in the era of personalized medicine. Ann. Allergy Asthma Immunol. 2018, 120, 350–353.
- van de Veen, W.; Akdis, M. The use of biologics for immune modulation in allergic disease. J. Clin. Investig. 2019, 129, 1452–1462.
- Agache, I.; Akdis, C.A.; Akdis, M.; Canonica, G.W.; Casale, T.; Chivato, T.; Corren, J.; Chu, D.K.; Del Giacco, S.; Eiwegger, T.; et al. EAACI Biologicals Guidelines—Recommendations for severe asthma. Allergy 2021, 76, 14–44.
- Nucala, INN-Mepolizumab (Europa.eu). Available online: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf (accessed on 23 October 2022).
- Kahn, J.-E.; Grandpeix-Guyodo, C.; Marroun, I.; Catherinot, E.; Mellot, F.; Roufosse, F.; Blétry, O. Sustained response to mepolizumab in refractory Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010, 125, 267–270.
- Kim, S.; Marigowda, G.; Oren, E.; Israel, E.; Wechsler, M.E. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J. Allergy Clin. Immunol. 2010, 125, 1336–1343.
- Moosig, F.; Gross, W.L.; Herrmann, K.; Bremer, J.P.; Hellmich, B. Targeting interleukin-5 in refractory and relapsing Churg-Strauss syndrome. Ann. Intern. Med. 2011, 155, 341–343.
- Herrmann, K.; Gross, W.L.; Moosig, F. Extended follow-up after stopping mepolizumab in relapsing/refractory Churg-Strauss syndrome. Clin. Exp. Rheumatol. 2012, 30 (Suppl. S70), S62–S65.
- Wechsler, M.E.; Akuthota, P.; Jayne, D.; Khoury, P.; Klion, A.; Langford, C.A.; Merkel, P.A.; Moosig, F.; Specks, U.; Cid, M.C.; et al. Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis. N. Engl. J. Med. 2017, 376, 1921–1932.
- Stone, J.H.; Hoffman, G.S.; Merkel, P.A.; Min, Y.I.; Uhlfelder, M.L.; Hellmann, D.B.; Specks, U.; Allen, N.B.; Davis, J.C.; Spiera, R.F.; et al. A disease-specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001, 44, 912–920.
- Ueno, M.; Miyagawa, I.; Nakano, K.; Iwata, S.; Hanami, K.; Fukuyo, S.; Kubo, S.; Miyazaki, Y.; Kawabe, A.; Yoshinari, H.; et al. Effectiveness and safety of mepolizumab in combination with corticosteroids in patients with eosinophilic granulomatosis with polyangiitis. Arthritis Res. Ther. 2021, 23, 86.
- Ueno, M.; Miyagawa, I.; Aritomi, T.; Kimura, K.; Iwata, S.; Hanami, K.; Fukuyo, S.; Kubo, S.; Miyazaki, Y.; Nakayamada, S.; et al. Safety and effectiveness of mepolizumab therapy in remission induction therapy for eosinophilic granulomatosis with polyangiitis: A retrospective study. Arthritis Res. Ther. 2022, 24, 159.
- Ríos-Garcés, R.; Prieto-González, S.; Hernández-Rodríguez, J.; Arismendi, E.; Alobid, I.; Penatti, A.E.; Cid, M.C.; Espígol-Frigolé, G. Response to mepolizumab according to disease manifestations in patients with eosinophilic granulomatosis with polyangiitis. Eur. J. Intern. Med. 2022, 95, 61–66.
- Kent, B.D.; d’Ancona, G.; Fernandes, M.; Green, L.; Roxas, C.; Thomson, L.; Nanzer, A.M.; Kavanagh, J.; Agarwal, S.; Jackson, D.J. Oral corticosteroid-sparing effects of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020, 6, 00311–2019.
- Available online: https://www.cinqairhcp.com/globalassets/cinqair-hcp-redesign/prescribing-information.pdf (accessed on 23 October 2022).
- Hellmich, B.; Flossmann, O.; Gross, W.L.; Bacon, P.; Cohen-Tervaert, J.W.; Guillevin, L.; Jayne, D.; Mahr, A.; Merkel, P.A.; Raspe, H.; et al. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: Focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann. Rheum. Dis. 2007, 66, 605–617.
- Manka, L.A.; Guntur, V.P.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Strand, M.J.; Wechsler, M.E. Efficacy and safety of reslizumab in the treatment of eosinophilic granulomatosis with polyangiitis. Ann. Allergy Asthma Immunol. 2021, 126, 696–701.e1.
- Koga, Y.; Aoki-Saito, H.; Kamide, Y.; Sato, M.; Tsurumaki, H.; Yatomi, M.; Ishizuka, T.; Hisada, T. Perspectives on the Efficacy of Benralizumab for Treatment of Eosinophilic Granulomatosis with Polyangiitis. Front. Pharmacol. 2022, 13, 865318.
- Guntur, V.P.; Manka, L.A.; Denson, J.L.; Dunn, R.M.; Dollin, Y.T.; Gill, M.; Kolakowski, C.; Strand, M.J.; Wechsler, M.E. Benralizumab as a Steroid-Sparing Treatment Option in Eosinophilic Granulomatosis with Polyangiitis. J. Allergy Clin. Immunol. Pract. 2021, 9, 1186–1193.e1.
- Padoan, R.; Chieco Bianchi, F.; Marchi, M.R.; Cazzador, D.; Felicetti, M.; Emanuelli, E.; Vianello, A.; Nicolai, P.; Doria, A.; Schiavon, F. Benralizumab as a glucocorticoid-sparing treatment option for severe asthma in eosinophilic granulomatosis with polyangiitis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3225–3227.e2.
- Nanzer, A.M.; Dhariwal, J.; Kavanagh, J.; Hearn, A.; Fernandes, M.; Thomson, L.; Roxas, C.; Green, L.; D’Ancona, G.; Agarwal, S.; et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020, 6, 00451–2020.
- Available online: https://clinicaltrials.gov/ct2/show/NCT04157348 (accessed on 23 October 2022).




