Eosinophilic granulomatosis with polyangiitis (EGPA) is a systemic disorder characterized by peripheral eosinophilia, severe eosinophilic asthma, sinusitis, transient pulmonary infiltrates, and features of medium/small-vessel vasculitis. EGPA belongs to the group of anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitides, although only 30 to 40% of patients display ANCA positivity, which is mainly of myeloperoxidase (MPO) specificity. Particularly, ANCA-positive patients typically show vasculitic features. Interleukin (IL)-5 has been demonstrated to play a crucial role in determining eosinophilic airway inflammation in EGPA patients. Specifically, maturation, activation, and survival of eosinophils especially depend on IL-5 availability. Therefore, blocking IL-5 biological activity may be a rewarding strategy for control of eosinophilic inflammation. Several monoclonal antibodies with the ability to interfere with the biological activity of IL-5 have been developed, namely, mepolizumab, reslizumab, and benralizumab. Here, we discuss the role of these drugs in the management of severe eosinophilic asthma in the context of EGPA and report the outcome of two EGPA patients with severe eosinophilic asthma treated at our outpatient clinic.
- eosinophilic granulomatosis with polyangiitis
- EGPA
- Churg–Strauss syndrome
- mepolizumab
- benralizumab
- interleukin-5
- IL-5
- severe eosinophilic asthma
1. Introduction
2. Therapeutic Management of EGPA
2.1. Standard Therapy
The cornerstone of therapy for EGPA remission induction is represented by glucocorticoids. Immunosuppressants are usually added to the glucocorticoid regimen, particularly in case of life-threatening organ involvement [12], which may be assessed with the aid of the five factor score (FFS), a prognostic tool proposed in 1996 [13] and revised in 2011 [14] (Table 1). FFS has been shown to be useful for survival prediction in EGPA; however, the original 1996 items have been refined in 2011 based on a better knowledge of the disease outcome [13][14][13,14]. Using the original set of items (1996), mortality at 5 years was observed to be 11.9% in the absence of any prognostic factor; with 1 of the 5 factors present, mortality increased to 25.9%, while with 2 or more of the 5 factors present (FFS = 2, see explanation in Table 1), mortality was shown to loom over 45.95% of the patients. In the 2011 update, age ≥ 65 years was also recognized as an independent predictor of poor prognosis, while visceral involvement was retained because it was still found to heavily weigh on the outcome. Conversely, ear, nose, and throat symptoms were associated with a lower relative risk of death. According to the 2011 set of criteria, mortality at 5 years was as follows: 9%, 21%, and 40% with FFS = 0, 1, and 2, respectively. Hence, the FFS may help identify patients requiring more aggressive treatment.| Original 1996 FFS | Revised 2011 FFS |
|---|---|
| Cardiac involvement Gastrointestinal disease (bleeding, perforation, infarction, or pancreatitis) Renal insufficiency (plasma creatinine concentration >1.6 mg/dL [141 mmol/L]) Proteinuria (>1 g/day) Central nervous system involvement |
Age > 65 years Cardiac insufficiency Renal insufficiency (stabilized peak creatinine 1.7 mg/dL [150 micromol/L]) Gastrointestinal involvement Absence of ENT manifestations (presence is associated with a better prognosis) |
2.2. Anti-IL-5-Targeted Therapies
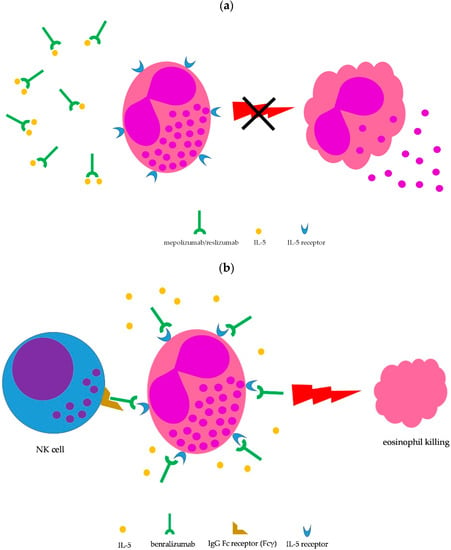
Lung involvement is not included among the factors defining severe EGPA (Table 1); however, the lung is the most commonly involved organ in EGPA (≥90% of cases) [1][2][8][11][1,2,8,11]. Moderate-to-severe asthma is the main clinical manifestation and chest X-ray may show transient pulmonary infiltrates, which are due to the accumulation of eosinophils. Upper airway involvement is also common, in the form of chronic rhinosinusitis with nasal polyps [1][2][8][1,2,8]. Asthma is managed according to international guidelines [16]; however, the severity of symptoms may require prolonged courses with oral steroids, which are difficult to taper. Therefore, IL-5-targeted therapy may be implemented to spare glucocorticoid side effects. To date, therapeutic monoclonal antibodies capable to interfere with the IL-5 pathway and, consequently, on eosinophil growth, recruitment, activation, and survival, include mepolizumab, reslizumab, and benralizumab [17][18][17,18]. Mepolizumab and reslizumab act by sequestering soluble IL-5, making it not available to eosinophils, whereas benralizumab binds to the IL-5 receptor, thus blocking access to IL-5, but is also able to induce eosinophil killing by natural killer cells through antibody-dependent cell toxicity (ADCC) (Figure 13) [17][18][17,18]. All three monoclonal antibodies have been approved for treatment of moderate-to-severe eosinophilic asthma as add-on therapy in patients unsatisfactorily controlled by standard therapy [19], thus being theoretically administrable in EGPA-associated eosinophilic asthma as well.