Eosinophilic granulomatosis with polyangiitis (EGPA, previously known as Churg–Strauss syndrome) belongs to the group of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides, which also include granulomatosis with polyangiitis (GPA), formerly known as Wegener’s granulomatosis, and microscopic polyangiitis (MPA) [
1,
2]. In EGPA, ANCA specificity is mainly directed (~70% of cases) towards myeloperoxidase (MPO) and is typically associated with a perinuclear fluorescence pattern (p-ANCA) upon immunofluorescence staining of fixed neutrophils [
3]. However, rates of ANCA positivity in EGPA are lower than those reported in the other two conditions of the group (GPA and MPA), being around 30–40% [
1,
2]. In addition, the clinical features of ANCA-positive patients significantly differ from those of ANCA-negative patients, with vasculitic involvement, in the form of glomerulonephritis, mononeuritis and/or alveolar hemorrhage, more frequent in the former, and cardiomyopathy more prevalent in the latter [
1,
2,
4]. According to the 2012 revised Chapel Hill Consensus Conference (CHCC) definition of vasculitides, EGPA is an eosinophil-rich and necrotizing granulomatous inflammation often involving the respiratory tract, with necrotizing vasculitis predominantly affecting small to medium vessels [
5,
6] and is associated with asthma and eosinophilia. Since both vessel wall inflammation and eosinophilic infiltration are involved in the pathogenesis of organ damage, EGPA shares features of both systemic vasculitis and hypereosinophilic syndromes [
7]. Clinically, the course of EGPA follows a sequential pattern, with a prodromal phase characterized by late-onset asthma and other allergic-like features, an eosinophilic phase with blood and tissue eosinophilia, and, eventually, a vasculitic phase, dominated by purpura, mononeuritis, glomerulonephritis, and/or alveolitis [
1,
2,
8]. These phases may as well overlap. Patients may also complain of nonspecific constitutional symptoms, such as fever, fatigue, arthralgia, and weight loss. However, the time interval between onset of initial symptoms and the vasculitic phase may be rather long, thus delaying a correct diagnosis. More commonly, indeed, the onset of vasculitic manifestations (such as the appearance of mononeuritis multiplex, purpura, and glomerulonephritis), in a patient previously known to be affected by chronic rhinosinusitis, persistent moderate-to-severe asthma and eosinophilia, alerts the clinician to the diagnosis of EGPA [
9,
10,
11].
2. Therapeutic Management of EGPA
2.1. Standard Therapy
The cornerstone of therapy for EGPA remission induction is represented by glucocorticoids. Immunosuppressants are usually added to the glucocorticoid regimen, particularly in case of life-threatening organ involvement [
12], which may be assessed with the aid of the five factor score (FFS), a prognostic tool proposed in 1996 [
13] and revised in 2011 [
14] (
Table 1). FFS has been shown to be useful for survival prediction in EGPA; however, the original 1996 items have been refined in 2011 based on a better knowledge of the disease outcome [
13,
14]. Using the original set of items (1996), mortality at 5 years was observed to be 11.9% in the absence of any prognostic factor; with 1 of the 5 factors present, mortality increased to 25.9%, while with 2 or more of the 5 factors present (FFS = 2, see explanation in
Table 1), mortality was shown to loom over 45.95% of the patients. In the 2011 update, age ≥ 65 years was also recognized as an independent predictor of poor prognosis, while visceral involvement was retained because it was still found to heavily weigh on the outcome. Conversely, ear, nose, and throat symptoms were associated with a lower relative risk of death. According to the 2011 set of criteria, mortality at 5 years was as follows: 9%, 21%, and 40% with FFS = 0, 1, and 2, respectively. Hence, the FFS may help identify patients requiring more aggressive treatment.
Table 1. Comparison of 1996 and 2011 five factor score (FFS) items.
| Original 1996 FFS |
Revised 2011 FFS |
Cardiac involvement
Gastrointestinal disease (bleeding, perforation, infarction, or pancreatitis)
Renal insufficiency (plasma creatinine concentration >1.6 mg/dL [141 mmol/L])
Proteinuria (>1 g/day)
Central nervous system involvement |
Age > 65 years
Cardiac insufficiency
Renal insufficiency
(stabilized peak creatinine 1.7 mg/dL [150 micromol/L])
Gastrointestinal involvement
Absence of ENT manifestations (presence is associated with a better prognosis) |
Glucocorticoids are administered orally at a dose of 0.5–1.0 mg/kg of prednisone equivalent. EGPA patients with no unfavorable prognostic factors according to the FFS may obtain remission with glucocorticoid therapy only, albeit relapses may still occur [
12]. However, in case of impending organ- or life-threatening disease (
Table 1), intravenous (i.v.) boluses (e.g., methylprednisolone 500–1000 mg daily for 3–5 days) may be preferred over the oral route as initial therapy, followed by oral administration and progressive tapering, according to clinical response; cyclophosphamide or rituximab are usually added to the i.v. glucorticoid induction regimen [
12]. Cyclophosphamide may be administered either orally (1.5–2.0 mg/kg for up to 6 months) or intravenously (15 mg/kg fortnightly for the first 3 infusions, then every three weeks for at least 3 more doses), with the oral route associated with a slightly greater risk of bladder toxicity and the i.v. route associated with a slightly greater risk of relapse. Cardiac involvement or ≥2 organ involvement may favor the use of cyclophosphamide because of an earlier onset of action with respect to rituximab [
12]. Rituximab may be considered in ANCA-positive patients, in case of glomerulonephritis, or unsatisfactory response to cyclophosphamide [
12]. Ease of administration with the protocol borrowed from rheumatoid arthritis (1 g, 2 weeks apart) [
15] may favor rituximab over cyclophosphamide. Maintenance of remission is then attempted with methotrexate, azathioprine, or mycophenolate mofetil, while glucocorticoids are progressively tapered down [
12]. However, many patients experience relapses, particularly during the tapering of glucocorticoids.
2.2. Anti-IL-5-Targeted Therapies
Lung involvement is not included among the factors defining severe EGPA (
Table 1); however, the lung is the most commonly involved organ in EGPA (≥90% of cases) [
1,
2,
8,
11]. Moderate-to-severe asthma is the main clinical manifestation and chest X-ray may show transient pulmonary infiltrates, which are due to the accumulation of eosinophils. Upper airway involvement is also common, in the form of chronic rhinosinusitis with nasal polyps [
1,
2,
8]. Asthma is managed according to international guidelines [
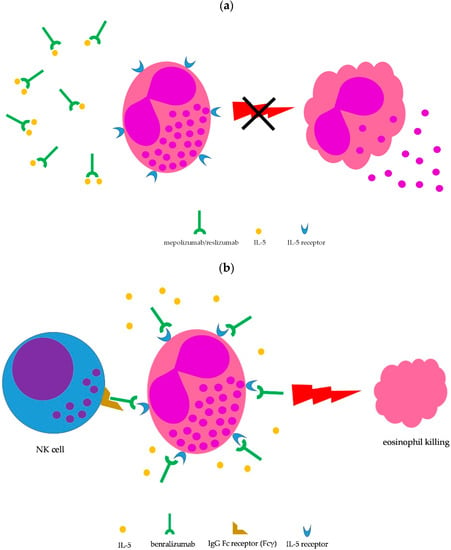
16]; however, the severity of symptoms may require prolonged courses with oral steroids, which are difficult to taper. Therefore, IL-5-targeted therapy may be implemented to spare glucocorticoid side effects. To date, therapeutic monoclonal antibodies capable to interfere with the IL-5 pathway and, consequently, on eosinophil growth, recruitment, activation, and survival, include mepolizumab, reslizumab, and benralizumab [
17,
18]. Mepolizumab and reslizumab act by sequestering soluble IL-5, making it not available to eosinophils, whereas benralizumab binds to the IL-5 receptor, thus blocking access to IL-5, but is also able to induce eosinophil killing by natural killer cells through antibody-dependent cell toxicity (ADCC) (
Figure 3) [
17,
18]. All three monoclonal antibodies have been approved for treatment of moderate-to-severe eosinophilic asthma as add-on therapy in patients unsatisfactorily controlled by standard therapy [
19], thus being theoretically administrable in EGPA-associated eosinophilic asthma as well.
Figure 3. Mechanism of action of IL-5-targeted monoclonal antibodies. (a) Blocking IL-5 by mepolizumab and reslizumab prevents eosinophil maturation, proliferation, recruitment, survival, priming, and activation (the latter shown here); (b) benralizumab binds to the IL-5 receptor on eosinophils, thus inhibiting cytokine-mediated cell stimulation; in addition, the Fc fragment of benralizumab is able to mediate eosinophil killing by NK cells through antibody-dependent cell cytotoxicity.
Moreover, apart from the upper and lower airways, eosinophils are also believed to play a crucial role in determining tissue damage in other typical manifestations of EGPA, such as peripheral neuritis and vasculitis [
7]. Therefore, IL-5-targeted therapies may also result in control of non-asthma features of EGPA.
2.2.1. Mepolizumab
To date, only mepolizumab has obtained indication for relapsing/remitting or refractory EGPA [
20]. In fact, following reports of encouraging results primarily in small series [
21,
22,
23,
24], a multicenter, double-blind, parallel-group, phase 3 randomized trial was eventually carried out to evaluate the efficacy and safety of mepolizumab in patients with refractory or relapsing EGPA on stable oral steroid treatment [
25]. Relapses considered by the investigators included active vasculitis (Birmingham Vasculitis Activity Score [BVAS] >0 [
26]), active asthma symptoms or signs, or active nasal or sinus disease, prompting an increase in the glucocorticoid dose, an initiation of or increase in immunosuppressive therapy, or hospitalization. Relapses could be of more than one type. The study confirmed the effectiveness of mepolizumab in maintaining remission throughout the study duration and in delaying major relapses when compared with placebo, with no significant between-group differences in adverse events [
25]. Specifically, the study met the two primary endpoints, i.e., (1) the accrued weeks of remission (28% vs. 3%, mepolizumab vs. placebo, respectively, with the treated group showing 24+ weeks of remission) plus prednisone daily dose ≤4 mg over 52 weeks (44% successfully lowering the dose to ≤4 mg and 18% discontinuing prednisone vs. 7% and 3%, mepolizumab vs. placebo, respectively) and (2) the proportion of patients in remission at weeks 36 and 48 (32% vs. 3%, mepolizumab vs. placebo, respectively). Rates of relapses were as follows (mepolizumab vs. placebo, respectively): vasculitis 43% vs. 65%; asthma 37% vs. 60%; sinusitis 35% vs. 51%. Overall, 20% of the patients had a vasculitic relapse only, while 54% had a combined vasculitic and asthmatic/sinonasal relapse. Finally, a subgroup analysis revealed that patients with an absolute eosinophil count greater than 150 cells/mm
3 at baseline were more likely to experience a greater efficacy when compared with patients with a lower value of eosinophils, who did not actually obtain a significant benefit from treatment [
25]. This observation clearly paves the way for a more effective selection of EGPA patients likely responding to IL-5 therapeutic inhibition in future studies. Therefore, based on these results, the Food and Drug Administration (FDA) licensed mepolizumab in the USA in 2017 as an add-on therapy with a steroid-sparing effect in adult patients with relapsing or refractory EGPA, at a dose of 300 mg subcutaneously (s.c.) every 4 weeks.
Subsequent reports from real-world clinical practice confirmed the safety and efficacy of mepolizumab in small series of patients with relapsing or refractory EGPA. Specifically, Ueno et al. [
27] treated 16 patients, all of them with comorbid asthma, for one year with the licensed 300 mg dose. At the 12-month assessment, mepolizumab treatment resulted in a decreased disease activity, a remission rate of 75%, a glucorticoid-sparing effect, and a reduced number of patients using concomitant immunosuppressants. The retention rate was excellent (100% of patients). Respiratory symptoms improved immediately after starting mepolizumab, with only 2 patients still complaining of chest discomfort at the 12-month evaluation. Only three patients had an infection. The same authors also retrospectively compared the effectiveness of mepolizumab (300 mg/monthly) with i.v. cyclophosphamide pulse therapy for remission induction in EGPA patients [
28]. Treatment was ≥6 months and both groups of patients were also administered high-dose steroids at the initiation of therapy. Even considering the critical issues of the study, mainly related to the small groups evaluated (7 patients in the mepolizumab group and 13 patients in the cyclophosphamide group), the results were promising: the retention rate for mepolizumab was again complete (100%), while only 61.5% of patients completed the pulse therapy (6 pulses total); adverse events were nearly twice as frequent in the cyclophosphamide group (53.8% vs. 28.6%, cyclophosphamide vs. mepolizumab, respectively). With regard to efficacy, there were no significant differences in BVAS and eosinophil count reductions between the two groups, however, mepolizumab-treated patients benefitted from a greater steroid dose reduction compared with cyclophosphamide-treated patients. Regarding lung involvement, chest manifestations decreased from 71.4% to 0% and from 58.3% to 8.3% at 6 months, mepolizumab vs. i.v. cyclophosphamide, respectively. Thus, mepolizumab for remission induction therapy in severe EGPA appeared to allow control of disease activity as effectively as cyclophosphamide, but with a greater reduction in concomitant steroid doses and fewer adverse events [
28]. Finally, a small series (11 EGPA patients, 3 with uncontrolled asthma), characterized by steroid dependence and unsatisfactory disease control, also achieved improvement of disease activity (no evidence of asthma and vasculitic manifestations at the last study follow-up) following treatment with mepolizumab [
29]. Again, the improvement in disease activity allowed notable glucocorticoid tapering.
2.2.2. Reslizumab
Fewer evidence is available for reslizumab as a controller drug in EGPA. A study involving nine patients with refractory, steroid-dependent EGPA patients with severe eosinophilic asthma were treated with reslizumab infusions at a dose of 3 mg/kg every 28 days [
37], according to the regimen approved as add-on therapy for treatment of severe asthma with an eosinophilic phenotype [
38]. Patients also had other EGPA features, namely, paranasal sinus disease (100% of patients), neurological involvement (44% of patients), and cardiac involvement (33% of patients). At the end of the 48 weeks of treatment, 7 out of 9 patients (~78%) had their steroid doses reduced to or below 7.5 mg/day (consistent with remission [
39]), while two patients were able to completely stop the drug. No increases in exacerbation frequency or hospitalization were seen and no treatment-limiting adverse effects were recorded. Since no significant improvement in overall BVAS scores was seen, the authors concluded that reslizumab may be less effective in controlling extrapulmonary manifestations of EGPA, namely, neuropathy, than in quenching activity of airway disease [
37]. Thus, reslizumab may be a therapeutic option in EGPA patients mainly troubled by severe eosinophilic asthma. Another open-label, pilot study involving 10 patients with EGPA [
40] demonstrated a significant reduction in the daily oral glucocorticoid dose after 7 monthly reslizumab treatments (equivalent to a 24-week treatment phase). Although only 6 of the 10 subjects completed all the study visits, 8 patients could be nonetheless analyzed; 5 out of these 8 patients were considered true responders to reslizimumab because of successful tapering of oral steroids, with no exacerbations during the treatment phase. Overall, three exacerbations were recorded during the treatment, and the most common was the worsening of asthma symptoms. One patient suffered from a severe adverse event, deemed to be causally linked with the study drug, requiring removal from the study [
40]. Because of the small numbers of patients and the design of these studies, randomized, controlled clinical trials are needed to validate the efficacy and safety of reslizumab for EGPA.
2.2.3. Benralizumab
Efficacy of benralizumab in EGPA is mainly supported by case reports [
41]. In addition, a small open-label pilot study including 10 EGPA patients suggested that benralizumab was effective in reducing both the daily dose of oral steroids and the exacerbation rate during treatment [
42]. Importantly, 8 of 10 subjects reached a minimum oral steroid dose of <5 mg daily, with 5 (50%) able to stop oral glucocorticoids after 40 weeks of treatment [
42]. Intriguingly, benralizumab has been reported to be effective even in EGPA patients failing previous treatment with mepolizumab or reslizumab. In 2 case studies (16 EGPA patients overall), including 6 patients failing previous treatment with mepolizumab and 1 patient failing both mepolizumab and reslizumab, benralizumab (30 mg s.c. every 4 weeks for the first 3 injections, then 30 mg s.c. every 8 weeks) significantly reduced oral glucocorticoid doses and improved both BVAS and ACT scores at the completion of the studies [
43,
44]. Complete depletion of peripheral eosinophils was seen in most of the patients, consistent with the drug’s mechanism of action (
Figure 3b). Currently, a phase three trial comparing benralizumab with mepolizumab in relapsing or refractory EGPA is ongoing (MANDARA trial, NCT04157348) [
45].