
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Helena Sá | -- | 5355 | 2022-10-19 15:55:01 | | | |
| 2 | Helena Sá | Meta information modification | 5355 | 2022-10-19 16:03:46 | | | | |
| 3 | Sirius Huang | Meta information modification | 5355 | 2022-10-20 06:45:05 | | |
Video Upload Options
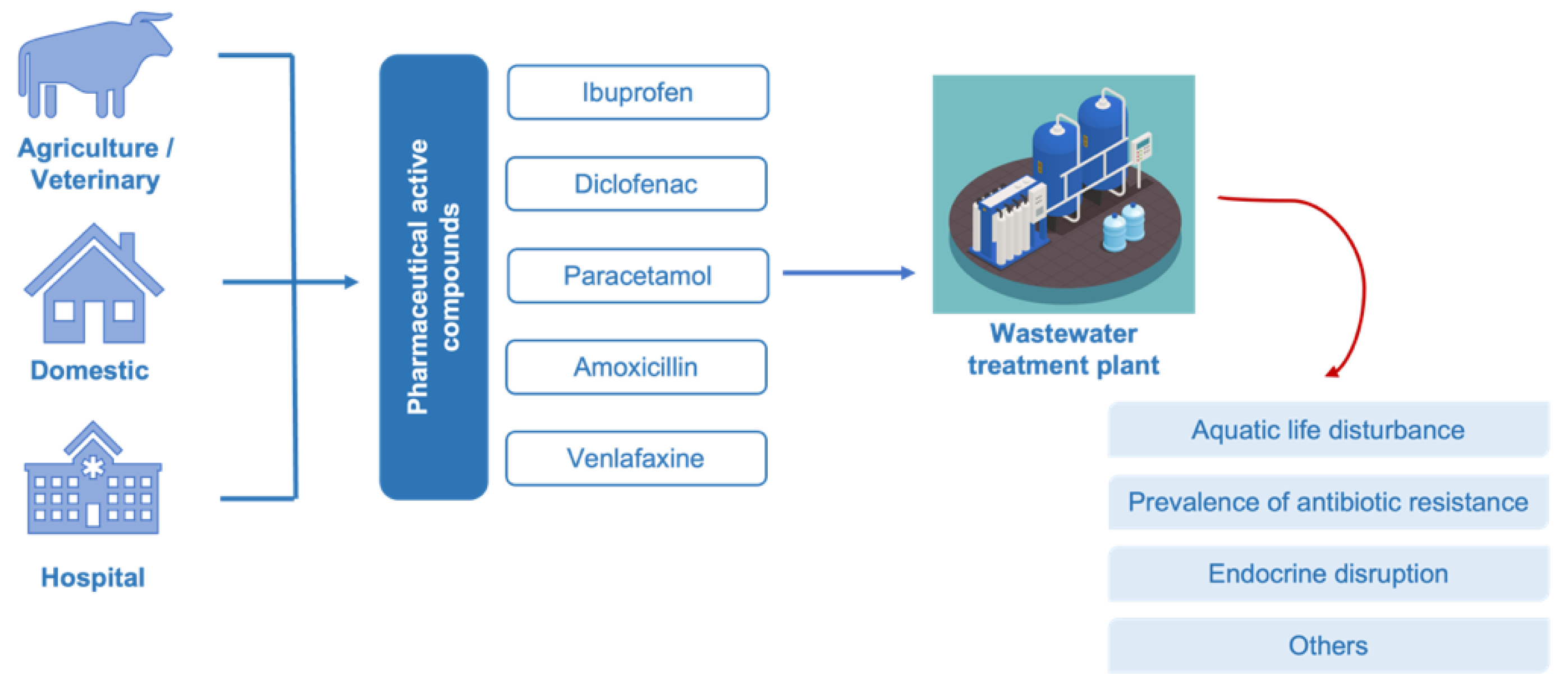
The worldwide access to pharmaceuticals and their continuous release into the environment have raised a serious global concern. Pharmaceuticals remain active even at low concentrations, therefore their occurrence in waterbodies may lead to successive deterioration of water quality with adverse impacts on the ecosystem and human health. To address this challenge, there is an evolving trend toward the search for effective methods to ensure efficient purification of both drinking water and wastewater. Biocatalytic transformation of pharmaceuticals using oxidoreductase enzymes, such as peroxidase and laccase, is a promising environmentally friendly solution for water treatment, where fungal species have been used as preferred producers due to their ligninolytic enzymatic systems.
1. Occurrence and Toxicity of Pharmaceutical Active Compounds
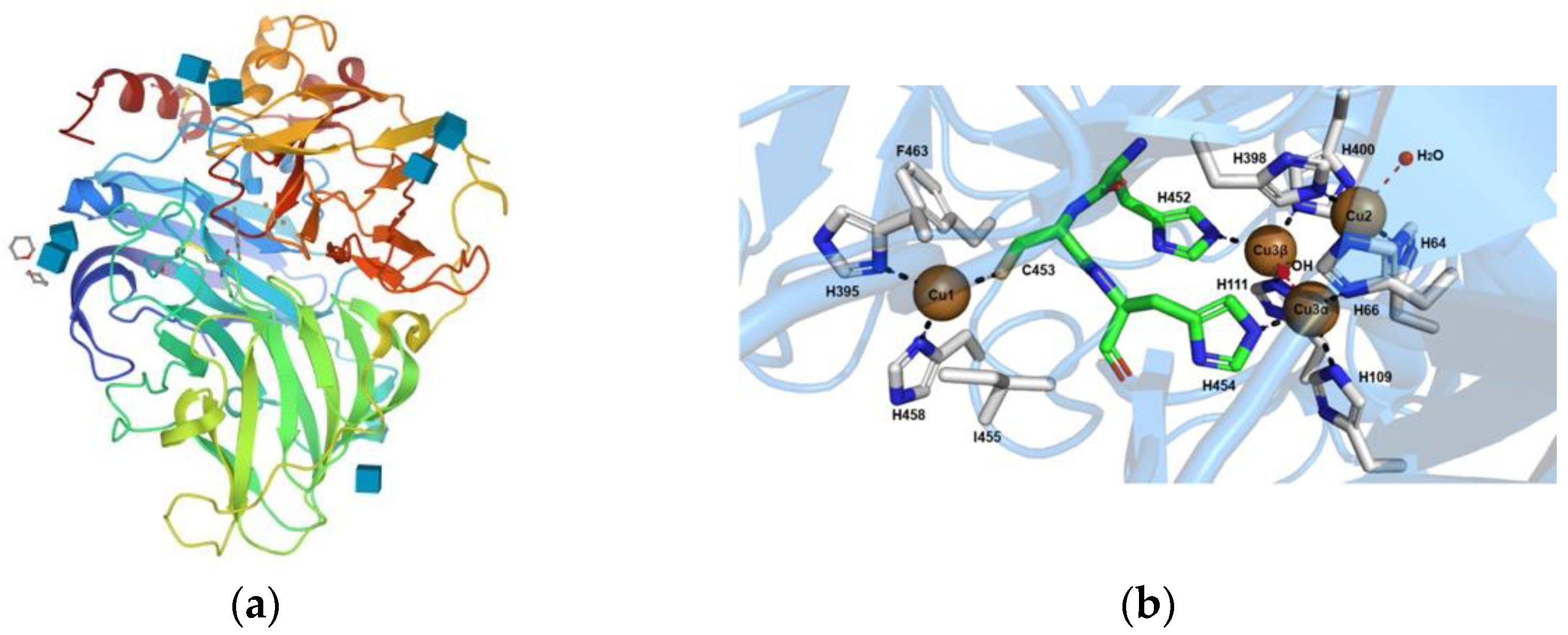
2. Enzymatic Biodegradation
| Compound | Enzyme | Source | PhAC (mg/L) | Reaction Conditions | Enzyme Load (U/L) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Diclofenac | LiP | Phanerochaete chrysosporium | 5 | pH 4, 24 mg/L H2O2, 25 °C, 2 h. | 180 | 100 | [34] |
| Lac | Trametes versicolor | 1 | pH 6.5, 25 °C, 5 h. | 500 | >90 | [35] | |
| Lac | Pycnoporus sanguineus | 100 | pH 5, 25 °C, 8 h. | 100 | 50 | [36] | |
| 5,7-Diiodo-8-hydroxyquinoline | Lac | Pycnoporus sanguineus | 100 | pH 5, 25 °C, 3.5 h. | 100 | 78 | [36] |
| Carbamazepine | Lac | Trametes versicolor | 1 | pH 6, 35 °C, 24 h. | 60 | 30 | [37] |
| Salicylic acid | Lac | Trametes pubescens | 0.001 | pH 6.9, 25 °C, 24 h. | 100 | >90 | [38] |
| 17-α-ethynyl estradiol | Lac | Trametes pubescens | 0.001 | pH 6.9, 25 °C, 24 h. | 100 | >90 | [38] |
| Sulfamethoxazole | Lac | Phanerochaete chrysosporium | 10 | pH 4.5, 30 °C, 48 h. | 6076 | 50 | [39] |
| Lac | Pycnoporus sanguineus | 10 | 30 °C, 72 h. | 170 | 29 | [40] | |
| 17-β-estradiol | Lac | Trametes hirsuta | 5 | pH 5, 25 °C, 120 min. | 5000 | 99 | [31] |
| Lac | Trametes pubescens | 0.001 | pH 6.9, 25 °C, 24 h. | 100 | >90 | [38] | |
| Tetracycline 1 Oxytetracycline 2 |
MnP | Phanerochaete chrysosporium | 50 | pH 4.8, 0.1 mM Mn2+, 0.1 mM H2O2, 37 °C, 4 h. | 40 | 73 1 84 2 |
[41] |
| Acetaminophen | Lac | Bjerkandera adusta TBB-03 | 20 | pH 5–7, 25 °C, 2 h. | 270 | 100 | [42] |
| HRP | Horseradish | 6 | pH 7.4, 400 μM H2O2, 25 °C, 4 h. | 12800 | 100 | [2] | |
| Triclosan | Lac | Trametes versicolor | 3 | pH 6, 25 °C, 4 h. | 2000 | 52 | [43] |
| Doxorubicin | Lac | Trametes Versicolor | 0.25 | pH 7, 30 °C, 2 h. | 900 | 100 | [44] |
| Imipramine | Lac | Paraconiothyrium variabile | 0.12 | pH 5, 37 °C, 6 h. | 1600 | 98 | [45] |
2.1. Laccase-Mediator Catalyzed System
| Oxidation Mechanism | Redox Mediator | Origin | Type of Mediator | Free Radical Generated |
|---|---|---|---|---|
| Electron transfer | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt | Synthetic | ABTS | ABTS•+ ABTS2+ |
| Hydrogen atom transfer | Hydroxybenzotriazole | Synthetic | N–OH | =N–O• Aminoxyl |
| N-hydroxyphthalimide | Synthetic | N–OH | =N–O• Aminoxyl |
|
| Violuric acid | Natural | N–OH | =N–O• Aminoxyl |
|
| Vanillin | Natural | C6H4(OH)(OCH3) | C6H5O• Phenoxyl |
|
| Syringaldehyde | Natural | C6H4(OH)(OCH3) | C6H5O• Phenoxyl |
|
| Acetosyringone | Natural | C6H4(OH)(OCH3) | C6H5O• Phenoxyl |
|
| p-coumaric acid | Natural | C6H4(OH)(OCH3) | C6H5O• Phenoxyl |
|
| Ionic oxidation | 2,2,6,6-tetramethylpiperidinyloxyl | Synthetic | N-O | N=O• Oxo-ammonium |
| Compound | Lac Source | Mediator | Reaction Conditions | Enzyme Load (U/L) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Diclofenac | Trametes versicolor | 1 mM of HBT 1 and SA 2 | 0.1 mg/L PhAC. 25 °C, 24 h. | 1440 | >95 1 80 2 |
[33] |
| Carbamazepine | Trametes versicolor | 0.018 mM of ABTS | 1 mg/L PhAC. pH 6, 35 °C, 24 h. | 60 | 95 | [37] |
| Sulfamethoxazole | Trametes versicolor | 0.5 mM ABTS, SA and AS | 20-25 mg/L PhAC. pH 6–7, 25 °C, 2–6 h. | 560 | 100 | [64] |
| Tetracycline Oxytetracycline |
Pycnoporus sp. SYBC-L10 | 1 mM of ABTS | 50 mg/L PhAC. pH 6, 5 min, 0 °C. | 10,000 | 100 | [22] |
| Chloramphenicol | Trametes hirsuta | 0.5 mM of SA, vanillin, ABTS and α-naphthol | 10 mg/L PhAC. pH 5, 25 °C, 48 h. | 50,220 | 100 | [32] |
| Ketoconazole | Trametes versicolor | 1 mM of HBT | 300 mg/L PhAC. pH 4.5, 45 °C, 6 h. | 1000 | 98 | [67] |
| Naproxen | Pleurotus ostreatus | 1 mM of ABTS | 0.5 mg/L PhAC. 25 °C, 8 h. | 0.26 | 80 | [66] |
| Olsalazine | Aspergillus aculeatus | 2 mM of ABTS 1, p-Coumaric acid 2 and HBT 3 | 100 mg/L PhAC. pH 5, 29 °C, 48 h. | - | 99 1 98 2 94 3 |
[68] |
| Atenolol | Trametes versicolor | 0.5 mM of TEMPO | 2.7 mg/L PhAC. pH 7, 25 °C, 4 h. | 5000 | 80 | [69] |
2.2. Transformation Mechanisms and Toxicity Evaluation
2.3. Immobilized Biocatalytic System
| Support Material | Enzyme | Immobilization Method | PhAC | Reaction Conditions | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| Poly(l-lactic acid)-co-poly(ε-caprolactone) nanofibers | Lac from Trametes versicolor | Encapsulation | Naproxen | 1 mg/L PhAC. pH 5, 25 °C, 24 h, 100 rpm. |
90 | [77] |
| Poly(l-lactic acid)-co-poly(ε-caprolactone) nanofibers | Lac from Trametes versicolor | Encapsulation | Diclofenac | 1 mg/L PhAC. pH 3, 25 °C, 24 h, 100 rpm. |
90 | [77] |
| Polyvinylidene fluoride membrane with multi-walled carbon nanotubes | Lac from Trametes hirsuta | Covalent bonding | 5 mg/L PhAC. pH 5, 25 °C, 4 h. |
95 | [78] | |
| Titania nanoparticles | Lac from Pycnoporus sanguineus CS43 | Covalent bonding | 10 mg/L PhAC. pH 4, 25 °C, 4 h. |
50 | [86] | |
| Micro-biochar from pine wood (PW) and pig manure (PM) | Lac from Trametes versicolor | Covalent bonding | 0.5 mg/L PhAC. pH 6.5, 25 °C, 5 h (PW) or 2 h (PM). |
99 | [82] | |
| Chitosan macro-beads | Lac from Trametes versicolor | Covalent bonding | 50 mg/L PhAC. pH 3, 25 °C, 4 h, 1:1 M ratio for ABTS:drug. |
90 | [30] | |
| CLEA | Lac from Trametes versicolor | Cross-linking | 0.001 mg/L PhAC. pH 5, 24 h, 22 °C. |
90 | [83] | |
| Polyacrylonitrile−biochar composite nanofibrous membrane |
Lac from Trametes versicolor | Covalent bonding | 0.2 mg/L PhAC, pH 4, 8 h, 35 °C. |
73 | [87] | |
| Polyacrylonitrile−biochar composite nanofibrous membrane |
Lac from Trametes versicolor | Covalent bonding | Chlortetracycline | 0.2 mg/L PhAC, pH 4, 8 h, 35 °C. |
63 | [87] |
| Polyvinylidene fluoride membrane with multi-walled carbon nanotubes | Lac from Trametes hirsuta | Covalent bonding | Carbamazepine | 5 mg/L PhAC. pH 5, 25 °C, 48 h. |
27 | [78] |
| Magnetite nanoparticles |
HRP LiP |
Adsorption | 0.35 mg/L PhAC. pH 3, 55 °C, 3 days. |
100 | [88] | |
| Polyimide aerogels | Lac from Trametes versicolor | Covalent bonding | 0.02 mg/L PhAC. pH 3, 25 °C, 24 h, 200 rpm. |
74 | [89] | |
| Pinewood nanobiochar | Lac from Trametes versicolor | Adsorption | 0.02 mg/L PhAC. pH 3.5, 25 °C, 24 h, 200 rpm. |
80 | [76] | |
| Polyamide/polyethylenimine nanofibers | Lac from Trametes versicolor | Covalent bonding | Triclosan | 10 mg/L PhAC. pH 7, 25 °C, 20 h, 80 rpm. |
74 | [90] |
| Titania nanoparticles | Lac from Pycnoporus sanguineus CS43 | Covalent bonding | Acetaminophen | 10 mg/L PhAC. pH 4, 25 °C, 4 h. |
90 | [86] |
| Commercial silica gel particles | Lac from Trametes versicolor | Covalent bonding | Sulfamethoxazole | 20 mg/L PhAC. pH 7, 25 °C, 0.5 h. 520 μM of ABTS. | 53 | [91] |
| Commercial silica gel particles | Lac from Trametes versicolor | Covalent bonding | Amoxicillin | 20 mg/L PhAC. pH 7, 25 °C, 4 h. 520 μM of ABTS. | 80 | [91] |
| M-CLEA | Lac from Cerrena unicolor | Cross-linking | Tetracycline | 100 mg/L PhAC. pH 6, 25 °C, 48 h. |
100 | [73] |
| Mesostructured cellular foam | Lac from Trametes versicolor | Adsorption | 1 mg/L PhAC. pH 5, 25 °C, 1 h. |
100 | [92] | |
| Bentonite-derived mesoporous materials | Lac from Trametes versicolor | Adsorption | 10 mg/L PhAC. 30 °C, 3 h. | 60 | [84] | |
| Cellulose beads |
Lac from Trametes versicolor | Covalent bonding | Indole | 15 mg/L PhAC. pH 5, 30 °C, 18 h. |
100 | [93] |
| Polypropylene beads | Lac from Myceliophthora thermophila | Adsorption | Morphine | 1 mg/L PhAC. pH 6, 25 °C, 0.5 h. |
100 | [94] |
| Graphene oxide and alginate matrix | Lac from Aspergillus niger | Adsorption/entrapment | Cetirizine dihydrochloride | 20 mg/L PhAC. pH 4.5, 25 °C, 1 h. |
100 | [95] |
| Pristine few layers graphene | Lac from Trametes versicolor | Adsorption | Labetalol hydrochloride | 1 mg/L PhAC pH 7, 25 °C, 1.5 h. 5 μM of ABTS. | 100 | [96] |
References
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in Freshwater Aquatic Environments: A Comparison of the African and European Challenge. Sci. Total Environ. 2019, 654, 324–337.
- Stadlmair, L.F.; Letzel, T.; Drewes, J.E.; Graßmann, J. Mass Spectrometry Based in Vitro Assay Investigations on the Transformation of Pharmaceutical Compounds by Oxidative Enzymes. Chemosphere 2017, 174, 466–477.
- dos Santos, C.R.; Arcanjo, G.S.; de Souza Santos, L.V.; Koch, K.; Amaral, M.C.S. Aquatic Concentration and Risk Assessment of Pharmaceutically Active Compounds in the Environment. Environ. Pollut. 2021, 290, 118049.
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, Fate and Removal of Pharmaceutically Active Compounds (PhACs) in Water and Wastewater Treatment Plants—A Review. J. Water Process. Eng. 2019, 32, 100927.
- Dey, S.; Bano, F.; Malik, A. Pharmaceuticals and Personal Care Product (PPCP) Contamination-a Global Discharge Inventory. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Vara Prasad, M.N., Vithanage, M., Kapley, A., Eds.; Emerging Contaminants and Micro Pollutants: New Delhi, India, 2019; pp. 1–26. ISBN 9780128161890.
- Yang, Y.Y.; Zhao, J.L.; Liu, Y.S.; Liu, W.R.; Zhang, Q.Q.; Yao, L.; Hu, L.X.; Zhang, J.N.; Jiang, Y.X.; Ying, G.G. Pharmaceuticals and Personal Care Products (PPCPs) and Artificial Sweeteners (ASs) in Surface and Ground Waters and Their Application as Indication of Wastewater Contamination. Sci. Total Environ. 2018, 616–617, 816–823.
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780.
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The Potential Implications of Reclaimed Wastewater Reuse for Irrigation on the Agricultural Environment: The Knowns and Unknowns of the Fate of Antibiotics and Antibiotic Resistant Bacteria and Resistance Genes–A Review. Water Res. 2017, 123, 448–467.
- O’Flaherty, E.; Cummins, E. Antibiotic Resistance in Surface Water Ecosystems: Presence in the Aquatic Environment, Prevention Strategies, and Risk Assessment. Hum. Ecol. Risk Assess. 2017, 23, 299–322.
- Tijani, J.O.; Fatoba, O.O.; Babajide, O.O.; Petrik, L.F. Pharmaceuticals, Endocrine Disruptors, Personal Care Products, Nanomaterials and Perfluorinated Pollutants: A Review. Environ. Chem. Lett. 2016, 14, 27–49.
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602.
- Commission Implementing Decision (EU) 2020/1161 of 4 August 2020 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. Available online: http://data.europa.eu/eli/dec_impl/2020/1161/oj (accessed on 29 April 2021).
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of Pharmaceutical Compounds and Other Organic Wastewater Contaminants in a Conventional Drinking-Water-Treatment Plant. Sci. Total Environ. 2004, 329, 99–113.
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990.
- Bilal, M.; Rasheed, T.; Nabeel, F.; Iqbal, H.M.N.; Zhao, Y. Hazardous Contaminants in the Environment and Their Laccase-Assisted Degradation–A Review. J. Environ. Manag. 2019, 234, 253–264.
- Stadlmair, L.F.; Letzel, T.; Drewes, J.E.; Grassmann, J. Enzymes in Removal of Pharmaceuticals from Wastewater: A Critical Review of Challenges, Applications and Screening Methods for Their Selection. Chemosphere 2018, 205, 649–661.
- Zdarta, J.; Meyer, A.S.; Jesionowski, T.; Pinelo, M. Developments in Support Materials for Immobilization of Oxidoreductases: A Comprehensive Review. Adv. Colloid Interface Sci. 2018, 258, 1–20.
- Bilal, M.; Lam, S.S.; Iqbal, H.M.N. Biocatalytic Remediation of Pharmaceutically Active Micropollutants for Environmental Sustainability. Environ. Pollut. 2022, 293, 118582.
- Peralta, R.M.; da Silva, B.P.; Gomes Côrrea, R.C.; Kato, C.G.; Vicente Seixas, F.A.; Bracht, A. Enzymes from Basidiomycetes-Peculiar and Efficient Tools for Biotechnology. In Biotechnology of Microbial Enzymes; Brahmachari, G., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 119–149. ISBN 9780128037461.
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic Treatment and Subsequent Toxicity of Organic Micropollutants Using Oxidoreductases-A Review. J. Clean. Prod. 2019, 221, 306–322.
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572.
- Tian, Q.; Dou, X.; Huang, L.; Wang, L.; Meng, D.; Zhai, L.; Shen, Y.; You, C.; Guan, Z.; Liao, X. Characterization of a Robust Cold-Adapted and Thermostable Laccase from Pycnoporus sp. SYBC-L10 with a Strong Ability for the Degradation of Tetracycline and Oxytetracycline by Laccase-Mediated Oxidation. J. Hazard. Mater. 2020, 382, 121084.
- Monssef, R.; Hassan, E.; Ramadan, E. Production of Laccase Enzyme for Their Potential Application to Decolorize Fungal Pigments on Aging Paper and Parchment. Ann. Agric. Sci. 2016, 61, 145–154.
- Wang, K.; Huang, K.; Jiang, G. Enhanced Removal of Aqueous Acetaminophen by a Laccase-Catalyzed Oxidative Coupling Reaction under a Dual-PH Optimization Strategy. Sci. Total Environ. 2018, 616–617, 1270–1278.
- Jones, S.M.; Solomon, E.I. Electron Transfer and Reaction Mechanism of Laccases. Cell. Mol. Life Sci. 2015, 72, 869–883.
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A Green Catalyst for the Biosynthesis of Poly-Phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307.
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C.N.; et al. Laccases: Structure, Function, and Potential Application in Water Bioremediation. Microb. Cell. Fact. 2019, 18, 200.
- Rodgers, C.J.; Blanford, C.F.; Giddens, S.R.; Skamnioti, P.; Armstrong, F.A.; Gurr, S.J. Designer Laccases: A Vogue for High-Potential Fungal Enzymes? Trends Biotechnol. 2010, 28, 63–72.
- Zimbardi, A.L.R.L.; Camargo, P.F.; Carli, S.; Neto, S.A.; Meleiro, L.P.; Rosa, J.C.; De Andrade, A.R.; Jorge, J.A.; Furriel, R.P.M. A High Redox Potential Laccase from Pycnoporus Sanguineus RP15: Potential Application for Dye Decolorization. Int. J. Mol. Sci. 2016, 17, 672.
- Apriceno, A.; Astolfi, M.L.; Girelli, A.M.; Scuto, F.R. A New Laccase-Mediator System Facing the Biodegradation Challenge: Insight into the NSAIDs Removal. Chemosphere 2019, 215, 535–542.
- Sun, K.; Cheng, X.; Yu, J.; Chen, L.; Wei, J.; Chen, W.; Wang, J.; Li, S.; Liu, Q.; Si, Y. Isolation of Trametes Hirsuta La-7 with High Laccase-Productivity and Its Application in Metabolism of 17β-Estradiol. Environ. Pollut. 2020, 263, 114381.
- Navada, K.K.; Kulal, A. Enzymatic Degradation of Chloramphenicol by Laccase from Trametes Hirsuta and Comparison among Mediators. Int. Biodeterior. Biodegrad. 2019, 138, 63–69.
- Nguyen, L.; Hai, F.; Kang, J.; Leusch, F.; Roddick, F.; Magram, S.; Price, W.; Nghiem, L. Enhancement of Trace Organic Contaminant Degradation by Crude Enzyme Extract from Trametes Versicolor Culture: Effect of Mediator Type and Concentration. J. Taiwan Inst. Chem. Eng. 2014, 45, 1855–1862.
- Zhang, Y.; Geißen, S.U. In Vitro Degradation of Carbamazepine and Diclofenac by Crude Lignin Peroxidase. J. Hazard. Mater. 2010, 176, 1089–1092.
- Lonappan, L.; Rouissi, T.; Laadila, M.A.; Brar, S.K.; Hernandez Galan, L.; Verma, M.; Surampalli, R.Y. Agro-Industrial-Produced Laccase for Degradation of Diclofenac and Identification of Transformation Products. ACS Sustain. Chem. Eng. 2017, 5, 5772–5781.
- Rodríguez-Delgado, M.; Orona-Navar, C.; García-Morales, R.; Hernandez-Luna, C.; Parra, R.; Mahlknecht, J.; Ornelas-Soto, N. Biotransformation Kinetics of Pharmaceutical and Industrial Micropollutants in Groundwaters by a Laccase Cocktail from Pycnoporus Sanguineus CS43 Fungi. Int. Biodeterior. Biodegrad. 2016, 108, 34–41.
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Biotransformation of Carbamazepine by Laccase-Mediator System: Kinetics, by-Products and Toxicity Assessment. Process. Biochem. 2018, 67, 147–154.
- Spina, F.; Cordero, C.; Schilirò, T.; Sgorbini, B.; Pignata, C.; Gilli, G.; Bicchi, C.; Varese, G.C. Removal of Micropollutants by Fungal Laccases in Model Solution and Municipal Wastewater: Evaluation of Estrogenic Activity and Ecotoxicity. J. Clean. Prod. 2015, 100, 185–194.
- Guo, X.L.; Zhu, Z.W.; Li, H.L. Biodegradation of Sulfamethoxazole by Phanerochaete Chrysosporium. J. Mol. Liq. 2014, 198, 169–172.
- Gao, N.; Liu, C.X.; Xu, Q.M.; Cheng, J.S.; Yuan, Y.J. Simultaneous Removal of Ciprofloxacin, Norfloxacin, Sulfamethoxazole by Co-Producing Oxidative Enzymes System of Phanerochaete Chrysosporium and Pycnoporus Sanguineus. Chemosphere 2018, 195, 146–155.
- Wen, X.; Jia, Y.; Li, J. Enzymatic Degradation of Tetracycline and Oxytetracycline by Crude Manganese Peroxidase Prepared from Phanerochaete Chrysosporium. J. Hazard. Mater. 2010, 177, 924–928.
- Kang, B.R.; Kim, S.Y.; Kang, M.; Lee, T.K. Removal of Pharmaceuticals and Personal Care Products Using Native Fungal Enzymes Extracted during the Ligninolytic Process. Environ. Res. 2021, 195, 110878.
- Sun, K.; Li, S.; Yu, J.; Gong, R.; Si, Y.; Liu, X.; Chu, G. Cu2+-Assisted Laccase from Trametes Versicolor Enhanced Self-Polyreaction of Triclosan. Chemosphere 2019, 225, 745–754.
- Kelbert, M.; Pereira, C.S.; Daronch, N.A.; Cesca, K.; Michels, C.; de Oliveira, D.; Soares, H.M. Laccase as an Efficacious Approach to Remove Anticancer Drugs: A Study of Doxorubicin Degradation, Kinetic Parameters, and Toxicity Assessment. J. Hazard. Mater. 2021, 409, 124520.
- Tahmasbi, H.; Khoshayand, M.R.; Bozorgi-Koushalshahi, M.; Heidary, M.; Ghazi-Khansari, M.; Faramarzi, M.A. Biocatalytic Conversion and Detoxification of Imipramine by the Laccase-Mediated System. Int. Biodeterior. Biodegrad. 2016, 108, 1–8.
- Elegbede, J.A.; Ajayi, V.A.; Lateef, A. Microbial Valorization of Corncob: Novel Route for Biotechnological Products for Sustainable Bioeconomy. Environ. Technol. Innov. 2021, 24, 102073.
- Pulicharla, R.; Das, R.K.; Kaur Brar, S.; Drogui, P.; Surampalli, R.Y. Degradation Kinetics of Chlortetracycline in Wastewater Using Ultrasonication Assisted Laccase. Chem. Eng. J. 2018, 347, 828–835.
- Patel, H.; Gupte, A. Optimization of Different Culture Conditions for Enhanced Laccase Production and Its Purification from Tricholoma Giganteum AGHP. Bioresour. Bioprocess. 2016, 3, 11.
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A Promising Laccase Immobilization Approach for Bisphenol A Removal from Aqueous Solutions. Bioresour. Technol. 2019, 271, 360–367.
- Karp, S.G.; Faraco, V.; Amore, A.; Birolo, L.; Giangrande, C.; Soccol, V.T.; Pandey, A.; Soccol, C.R. Characterization of Laccase Isoforms Produced by Pleurotus Ostreatus in Solid State Fermentation of Sugarcane Bagasse. Bioresour. Technol. 2012, 114, 735–739.
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Ding, Z. Laccase Production by Trametes Versicolor in Solid-State Fermentation Using Tea Residues as Substrate and Its Application in Dye Decolorization. J. Environ. Manag. 2020, 270, 110904.
- Omeje, K.O.; Nnolim, N.E.; Ezema, B.O.; Ozioko, J.N.; Eze, S.O.O. Synthetic Dyes Decolorization Potential of Agroindustrial Waste-Derived Thermo-Active Laccase from Aspergillus Species. Biocatal. Agric. Biotechnol. 2020, 29, 101800.
- Songulashvili, G.; Elisashvili, V.; Wasser, S.P.; Nevo, E.; Hadar, Y. Basidiomycetes Laccase and Manganese Peroxidase Activity in Submerged Fermentation of Food Industry Wastes. Enzyme Microb. Technol. 2007, 41, 57–61.
- Atilano-Camino, M.M.; Álvarez-Valencia, L.H.; García-González, A.; García-Reyes, R.B. Improving Laccase Production from Trametes Versicolor Using Lignocellulosic Residues as Cosubstrates and Evaluation of Enzymes for Blue Wastewater Biodegradation. J. Environ. Manag. 2020, 275, 111231.
- Li, G.; Liu, X.; Yuan, L. Improved Laccase Production by Funalia Trogii in Absorbent Fermentation with Nutrient Carrier. J. Biosci. Bioeng. 2017, 124, 381–385.
- Junior, J.A.; Vieira, Y.A.; Cruz, I.A.; da Silva Vilar, D.; Aguiar, M.M.; Torres, N.H.; Bharagava, R.N.; Lima, Á.S.; de Souza, R.L.; Romanholo Ferreira, L.F. Sequential Degradation of Raw Vinasse by a Laccase Enzyme Producing Fungus Pleurotus Sajor-Caju and Its ATPS Purification. Biotechnol. Rep. 2020, 25, e00411.
- Hultberg, M.; Ahrens, L.; Golovko, O. Use of Lignocellulosic Substrate Colonized by Oyster Mushroom (Pleurotus Ostreatus) for Removal of Organic Micropollutants from Water. J. Environ. Manage. 2020, 272, 111087.
- Xu, G.; Wang, J.; Yin, Q.; Fang, W.; Xiao, Y.; Fang, Z. Expression of a Thermo- and Alkali-Philic Fungal Laccase in Pichia Pastoris and Its Application. Protein Expr. Purif. 2019, 154, 16–24.
- Debaste, F.; Flahaut, S.; Penninckx, M.; Songulashvili, G. Chapter 15-Using Laccases for Food Preservation. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 501–541.
- Sondhi, S.; Sharma, P.; George, N.; Chauhan, P.S.; Puri, N.; Gupta, N. An Extracellular Thermo-Alkali-Stable Laccase from Bacillus Tequilensis SN4, with a Potential to Biobleach Softwood Pulp. 3 Biotech 2015, 5, 175–185.
- Yang, L.H.; Qiao, B.; Xu, Q.M.; Liu, S.; Yuan, Y.; Cheng, J.S. Biodegradation of Sulfonamide Antibiotics through the Heterologous Expression of Laccases from Bacteria and Investigation of Their Potential Degradation Pathways. J. Hazard. Mater. 2021, 416, 125815.
- Cañas, A.I.; Camarero, S. Laccases and Their Natural Mediators: Biotechnological Tools for Sustainable Eco-Friendly Processes. Biotechnol. Adv. 2010, 28, 694–705.
- Yang, S.; Hai, F.I.; Nghiem, L.D.; Price, W.E.; Roddick, F.; Moreira, M.T.; Magram, S.F. Understanding the Factors Controlling the Removal of Trace Organic Contaminants by White-Rot Fungi and Their Lignin Modifying Enzymes: A Critical Review. Bioresour. Technol. 2013, 141, 97–108.
- Margot, J.; Copin, P.J.; von Gunten, U.; Barry, D.A.; Holliger, C. Sulfamethoxazole and Isoproturon Degradation and Detoxification by a Laccase-Mediator System: Influence of Treatment Conditions and Mechanistic Aspects. Biochem. Eng. J. 2015, 103, 47–59.
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Removal of Pharmaceutical Compounds in Water and Wastewater Using Fungal Oxidoreductase Enzymes. Environ. Pollut. 2018, 234, 190–213.
- Ashe, B.; Nguyen, L.N.; Hai, F.I.; Lee, D.J.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E.; Nghiem, L.D. Impacts of Redox-Mediator Type on Trace Organic Contaminants Degradation by Laccase: Degradation Efficiency, Laccase Stability and Effluent Toxicity. Int. Biodeterior. Biodegrad. 2016, 113, 169–176.
- Yousefi-Ahmadipour, A.; Bozorgi-Koshalshahi, M.; Mogharabi, M.; Amini, M.; Ghazi-Khansari, M.; Faramarzi, M.A. Laccase-Catalyzed Treatment of Ketoconazole, Identification of Biotransformed Metabolites, Determination of Kinetic Parameters, and Evaluation of Micro-Toxicity. J. Mol. Catal. B Enzym 2016, 133, 77–84.
- Bankole, P.O.; Semple, K.T.; Jeon, B.H.; Govindwar, S.P. Impact of Redox-Mediators in the Degradation of Olsalazine by Marine-Derived Fungus, Aspergillus Aculeatus Strain Bpo2: Response Surface Methodology, Laccase Stability and Kinetics. Ecotoxicol. Environ. Saf. 2021, 208, 111742.
- Feng, Y.; Shen, M.; Wang, Z.; Liu, G. Transformation of Atenolol by a Laccase-Mediator System: Efficiencies, Effect of Water Constituents, and Transformation Pathways. Ecotoxicol. Environ. Saf. 2019, 183, 109555.
- Nguyen, L.; Hai, F.; Yang, S.; Kang, J.; Leusch, F.; Roddick, F.; Price, W.; Nghiem, L. Removal of Pharmaceuticals, Steroid Hormones, Phytoestrogens, UV-Filters, Industrial Chemicals and Pesticides by Trametes Versicolor: Role of Biosorption and Biodegradation. Int. Biodeterior. Biodegrad. 2014, 88, 169–175.
- Kózka, B.; Nałęcz-Jawecki, G.; Turło, J.; Giebułtowicz, J. Application of Pleurotus Ostreatus to Efficient Removal of Selected Antidepressants and Immunosuppressant. J. Environ. Manag. 2020, 273, 111131.
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Momba, M.N.B. Assessing a Co-Culture Fungal Granule Ability to Remove Pharmaceuticals in a Sequencing Batch Reactor. Environ. Technol. 2020, 43, 1684–1699.
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of Tetracycline by Immobilized Laccase and the Proposed Transformation Pathway. J. Hazard. Mater. 2017, 322, 525–531.
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly Laccases Treatment to Challenge Micropollutants Issue in Municipal Wastewaters. Environ. Pollut. 2020, 257, 113579.
- Becker, D.; Varela Della Giustina, S.; Rodriguez-Mozaz, S.; Schoevaart, R.; Barceló, D.; de Cazes, M.; Belleville, M.P.; Sanchez-Marcano, J.; de Gunzburg, J.; Couillerot, O.; et al. Removal of Antibiotics in Wastewater by Enzymatic Treatment with Fungal Laccase–Degradation of Compounds Does Not Always Eliminate Toxicity. Bioresour. Technol. 2016, 219, 500–509.
- Naghdi, M.; Taheran, M.; Brar, S.K.; Kermanshahi-pour, A.; Verma, M.; Surampalli, R.Y. Immobilized Laccase on Oxygen Functionalized Nanobiochars through Mineral Acids Treatment for Removal of Carbamazepine. Sci. Total Environ. 2017, 584–585, 393–401.
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust Biodegradation of Naproxen and Diclofenac by Laccase Immobilized Using Electrospun Nanofibers with Enhanced Stability and Reusability. Mater. Sci. Eng. C 2019, 103, 109789.
- Masjoudi, M.; Golgoli, M.; Ghobadi Nejad, Z.; Sadeghzadeh, S.; Borghei, S.M. Pharmaceuticals Removal by Immobilized Laccase on Polyvinylidene Fluoride Nanocomposite with Multi-Walled Carbon Nanotubes. Chemosphere 2021, 263, 128043.
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on Methods and Easy Separated Support Materials for Enzymes Immobilization. TrAC-Trends Anal. Chem. 2018, 102, 332–342.
- Nguyen, L.N.; Hai, F.I.; Dosseto, A.; Richardson, C.; Price, W.E.; Nghiem, L.D. Continuous Adsorption and Biotransformation of Micropollutants by Granular Activated Carbon-Bound Laccase in a Packed-Bed Enzyme Reactor. Bioresour. Technol. 2016, 210, 108–116.
- Fernández-Fernández, M.; Sanromán, M.Á.; Moldes, D. Recent Developments and Applications of Immobilized Laccase. Biotechnol. Adv. 2013, 31, 1808–1825.
- Lonappan, L.; Liu, Y.; Rouissi, T.; Pourcel, F.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Covalent Immobilization of Laccase on Citric Acid Functionalized Micro-Biochars Derived from Different Feedstock and Removal of Diclofenac. Chem. Eng. J. 2018, 351, 985–994.
- Primožič, M.; Kravanja, G.; Knez, Ž.; Crnjac, A.; Leitgeb, M. Immobilized Laccase in the Form of (Magnetic) Cross-Linked Enzyme Aggregates for Sustainable Diclofenac (Bio)Degradation. J. Clean. Prod. 2020, 275, 124121.
- Wen, X.; Zeng, Z.; Du, C.; Huang, D.; Zeng, G.; Xiao, R.; Lai, C.; Xu, P.; Zhang, C.; Wan, J.; et al. Immobilized Laccase on Bentonite-Derived Mesoporous Materials for Removal of Tetracycline. Chemosphere 2019, 222, 865–871.
- Daronch, N.A.; Kelbert, M.; Pereira, C.S.; de Araújo, P.H.H.; de Oliveira, D. Elucidating the Choice for a Precise Matrix for Laccase Immobilization: A Review. Chem. Eng. J. 2020, 397, 125506.
- García-Morales, R.; García-García, A.; Orona-Navar, C.; Osma, J.F.; Nigam, K.D.P.; Ornelas-Soto, N. Biotransformation of Emerging Pollutants in Groundwater by Laccase from P. Sanguineus CS43 Immobilized onto Titania Nanoparticles. J. Environ. Chem. Eng. 2018, 6, 710–717.
- Taheran, M.; Naghdi, M.; Brar, S.K.; Knystautas, E.J.; Verma, M.; Surampalli, R.Y. Covalent Immobilization of Laccase onto Nanofibrous Membrane for Degradation of Pharmaceutical Residues in Water. ACS Sustain. Chem. Eng. 2017, 5, 10430–10438.
- Pylypchuk, I.V.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A. Removal of Diclofenac, Paracetamol, and Carbamazepine from Model Aqueous Solutions by Magnetic Sol–Gel Encapsulated Horseradish Peroxidase and Lignin Peroxidase Composites. Nanomaterials 2020, 10, 282.
- Simón-Herrero, C.; Naghdi, M.; Taheran, M.; Kaur Brar, S.; Romero, A.; Valverde, J.L.; Avalos Ramirez, A.; Sánchez-Silva, L. Immobilized Laccase on Polyimide Aerogels for Removal of Carbamazepine. J. Hazard. Mater. 2019, 376, 83–90.
- Maryšková, M.; Schaabová, M.; Tománková, H.; Vít, N.; Miroslava, R. Wastewater Treatment by Novel Polyamide/Polyethylenimine Nanofibers with Immobilized Laccase. Water 2020, 12, 588.
- Guardado, A.L.P.; Druon-Bocquet, S.; Belleville, M.P.; Sanchez-Marcano, J. A Novel Process for the Covalent Immobilization of Laccases on Silica Gel and Its Application for the Elimination of Pharmaceutical Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 25579–25593.
- Zdarta, J.; Feliczak-Guzik, A.; Siwińska-Ciesielczyk, K.; Nowak, I.; Jesionowski, T. Mesostructured Cellular Foam Silica Materials for Laccase Immobilization and Tetracycline Removal: A Comprehensive Study. Microporous Mesoporous Mater. 2020, 291, 109688.
- Yaohua, G.; Ping, X.; Feng, J.; Keren, S. Co-Immobilization of Laccase and ABTS onto Novel Dual-Functionalized Cellulose Beads for Highly Improved Biodegradation of Indole. J. Hazard. Mater. 2019, 365, 118–124.
- Huber, D.; Bleymaier, K.; Pellis, A.; Vielnascher, R.; Daxbacher, A.; Greimel, K.J.; Guebitz, G.M. Laccase Catalyzed Elimination of Morphine from Aqueous Systems. New Biotechnol. 2018, 42, 19–25.
- Sharifi-Bonab, M.; Arjomandi Rad, F.; Talat Mehrabad, J. Preparation of Laccase-Graphene Oxide Nanosheet/Alginate Composite: Application for the Removal of Cetirizine from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 3013–3020.
- Dong, S.; Jing, X.; Cao, Y.; Xia, E.; Gao, S.; Mao, L. Non-Covalent Assembled Laccase-Graphene Composite: Property, Stability and Performance in Beta-Blocker Removal. Environ. Pollut. 2019, 252, 907–916.
- Jia, Y.; Chen, Y.; Luo, J.; Hu, Y. Immobilization of Laccase onto Meso-MIL-53(Al) via Physical Adsorption for the Catalytic Conversion of Triclosan. Ecotoxicol. Environ. Saf. 2019, 184, 109670.




