2. Enzymatic Biodegradation
Enzymes are catalysts that conduct, within the mild conditions of temperature and pH, chemical reactions at a remarkably high rate, efficiency and specificity [
22]. Several enzyme systems have been used for the efficient transformation and degradation of organic pollutants and have been shown to oxidize and degrade the pollutants into smaller intermediates [
35].
The literature survey shows that most enzymatic remediation studies use oxidoreductase enzymes [
36,
37]. These enzymes are largely produced by the white-rot fungi (WRF), as extracellular enzymes for the degradation of lignin. WRF and their ligninolytic enzymes, namely lignin peroxidase (LiP), manganese peroxidase (MnP), versatile peroxidase (VP) and Lac, have been demonstrated to be capable of transforming a wide range of compounds. This ability is a result of the structural similarities of several micropollutants to lignin, as well as the fact that ligninolytic enzymes are substrate-nonspecific [
38]. Other enzymes are, as well, used as biocatalysts for the remediation of toxic compounds, such as tyrosinase, horseradish peroxidase (HRP) and phenoloxidase [
36].
Peroxidases (EC 1.11.1.X) are a vast group of heme-containing oxidoreductases, which use hydrogen peroxide as an electron-acceptor to catalyze oxidative reactions [
39], whereas Lac (EC 1.10.3.2) are glycosylated multi-copper oxidases that catalyzes one-electron oxidation of various substrates associated with the reduction of molecular oxygen to water, via a radical-catalyzed reaction mechanism [
40]. In contrast to peroxidases that require the supply of hydrogen peroxide, Lac only requests oxygen as the final electron acceptor for the oxidation reaction to occur, offering an alternative green approach for the biodegradation of several PhAC [
41]. Lac is selective towards phenolic compounds, but due to the non-specificity, they are also able to degrade aromatic amines and related substances, thiol groups, diamines, N-heterocycles and phenothiazines [
42].
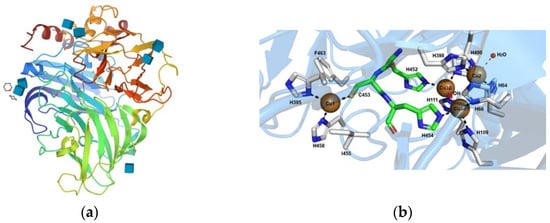
The active sites of Lac enzyme, as represented in
Figure 2, contain four copper ions: one type 1 (T1) copper ion and a trinuclear copper cluster (TNC) composed of one type 2 (T2) copper ion coupled to binuclear type 3 (T3) copper ions [
43]. The T1 copper site is the primary electron-acceptor for electrons offered by the substrate. The electrons are then moved to the TNC through highly conserved His-Cys-His tripeptide. After that, oxygen reacts with the fully reduced enzyme to form a peroxy-intermediate (PI), and then the PI is transformed back into a native intermediate, through a two-electron reduction. Once the fully reduced state is recovered, the final products are released from the TNC site. This results in the production of four radicals by the oxidation of four molecules of substrate, while one molecule of oxygen is reduced to two molecules of water and four protons (H
+) are consumed from the solution [
44,
45].
Figure 2. Lac from Trametes versicolor (Protein Data Bank code 1KYA) (
a). Representation of the different amino acids of the catalytic site that coordinates the T1, T2 and T3 copper sites (
b), reprinted from Ref. [
46].
The Lac and their active sites with copper ions play a key role in the reduction of oxygen. The redox potential (E°) difference between the T1 copper site and the substrate is one of the major factors that affect the oxidation rate. Accordingly, Lac are classified into three groups: low- (0.4–0.5 V), medium- (0.5–0.6 V) and high- (0.7–0.8 V) redox potential [
47,
48]. The variety of redox potentials is strictly related to the sources (e.g., bacterial, plant or fungi), due to the difference in the amino acid residues composition around the copper of the first reaction site. Lac from WRF has the highest redox potential, between 0.730 and 0.790 V, with phenylalanine as the non-coordinating axial ligand at the T1 copper site [
49]. Among fungal species, Lac have been comprehensively identified from
Ascomycetes and
Basidiomycetes. Nonetheless, white-rot basidiomycetes such as
Trametes versicolor,
Pleurotus ostreatus or
Cerrena unicolor, are noted for being efficient lignin degraders and Lac producers [
36,
50].
Several studies using enzyme extracts have been conducted for biodegradation of PhAC (
Table 1). Although high efficiency rates are presented, the enzymatic wastewater treatment process is still relatively expensive, mainly due to the cost of commercial enzymes. One potential solution that may get the process cheaper is the application of crude enzymatic extracts. This might positively influence Lac catalytic activity and stability, as well as avoid the costly process of enzyme purification [
39,
51]. Furthermore, the crude extract offers efficient oxidation, since the synergistic action of the enzymes and a host of natural mediators and co-factors secreted by the fungi can potentially enhance the general performance [
52].
Table 1. Examples of biodegradation of pharmaceutical micropollutants by enzymes.
A huge amount of renewable biomass is generated annually due to agricultural, food and industrial activities. Agricultural and food wastes are often disposed of indiscriminately or burnt off, thereby constituting environmental hazards and contributing to global warming through the generation of greenhouse gases. To achieve environmental sustainability, value-addition to wastes and promotion of advances in the circular economy, agro-food wastes are now being investigated for valorization through bio-enzymatic approach [
63]. For fungal cultures, a wide variety of lignocellulosic biomass has been used as an alternative substrate in submerged fermentation, solid-state fermentation or semisolid-state fermentation. Peanut shell, wheat straw, wheat bran, rice straw, rice bran, agave bagasse, sugarcane vinasse, corn bran, fruit peel, tea, sunflower seeds and apple pomace are some of the studied lignocellulosic wastes for Lac production for water remediation [
64,
65,
66,
67,
68,
69,
70,
71,
72,
73].
Lonappan et al. [
54] present a novel insight on residue valorization and found that apple pomace and pulp and paper solid waste were capable of inducing Lac production by
T. versicolor. The resulting crude enzyme was effective in the degradation of diclofenac sodium (DCF) at an environmentally relevant concentration (0.5 mg/L).
Kang et al. [
61] stimulated the production of
Bjerkandera adusta TBB-03 Lac using lignocellulosic substrates as the sole enzyme inducer (e.g., ash wood chips). The formed Lac consistently showed a high capability to degrade acetaminophen (APAP) under various conditions. The authors also defended a 22% of improvement in SMX removal in the presence of APAP, that could act as mediator for oxidation. Enzyme suspension with lower purification levels may contain phenolic compounds that act as natural mediators and consequently increase removal efficiency. For instance, Lac from a mushroom substrate, based on sawdust and wheat bran, colonized by
P. ostreatus, demonstrates significant removal of DCF (90%), bicalutamide (43%) lamotrigine (73%) and metformin (49%), when compared with the control with an uncolonized mushroom substrate [
74].
Most Lac used to degrade pharmaceuticals to date are mainly sourced from fungi. However, heterologous expression of Lac genes has attracted increasing attention due to the requirement to find more specific enzymes tailored to the complexity of wastewater matrices [
75]. Protein engineering may provide higher enzyme yields and may allow the production of Lac with improved properties such as operational stability, pH stability, solvent stability and thermostability [
76,
77]. For example, a novel Lac derived from
Bacillus tequilensis SN4 was demonstrated to be thermo-alkali-stable [
77]. Lac Lcc9 from
Coprinopsis cinerea expressed in
Pichia pastoris also presented improved activity and stability at neutral and alkaline pH conditions [
75]. Moreover, heterologous expressed bacterial Lac in
Escherichia coli successfully degraded sulfanilamide antibiotics [
78].
2.1. Laccase-Mediator Catalyzed System
Lac possess a relatively low redox potential (≤0.8 V) compared to peroxidase (>1 V). The redox potential difference between the substrate and the enzyme T1 copper affects the oxidative capacity of Lac. For example, Lac efficiently promotes single-electron oxidation of phenols. Accordingly, non-phenolic compounds, with redox potential above 1.3 V, are not directly prone to oxidation by Lac [
79]. This lack of affinity of Lac is generally influenced by the distribution of functional groups in the chemical structure of the substrates. Compounds with electron withdrawing groups (EWG) such as carboxylic (-COOH), amide (-CONR
2), halogen (-X) and nitro (-NO
2) have lower enzyme affinity due to the less susceptible to oxidative catabolism [
80].
The degradation efficiency of pollutants using Lac can be enhanced by the addition of redox mediators that are easily oxidized by the enzyme to free radicals. The presence of these small molecular weight compounds allows Lac to overcome a kinetic barrier and increase the spectrum of pollutants potentially degraded, as the mediator species have higher redox potentials. These compounds act as an “electron shuttle”, enabling the oxidation of complex substrates by highly reactive radicals that result from mediator oxidation by Lac. These radicals may return to their parent compound through reduction during the oxidation of the target pollutant [
52,
81].
Table 2 presents a list of redox mediators and their related information. The hydrogen atom transfer (HAT), electron transfer (ET) and ionic mechanisms are the primary mechanisms for mediator oxidation of a compound. The oxidation mechanism of the 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 2, 2, 6, 6-tetramethyl-1-piperidinyloxy (TEMPO) radicals follows ET and ionic mechanisms, respectively. As well as
p-coumaric acid, hydroxybenzotriazole (HBT), N-hydroxyphthalimide (HPI) and syringaldehyde (SA) follow HAT oxidation mechanism [
18].
Table 2. Characteristics of redox mediators used in treatment of micropollutants by Lac-mediator systems.
The mediator type and concentration have a major impact on the practicality and feasibility of using Lac in PhAC degradation. Some literature studies that used mediators coupled with enzymes for pharmaceutical transformation are presented in
Table 3. Naproxen (NPX) is an example of a recalcitrant drug able to resist enzymatic oxidation due to the presence of the EWG carboxyl and the absence of any strong electron-donating groups (EDG). Lac mediated with HBT achieved the greatest removal of NPX (80%) at the highest mediator concentration of 1 mM, while the performance of violuric acid (VLA), another N-OH type mediator, was weaker (~60%). At an inferior dose (0.5 mM), HBT and ABTS mediators still presented very efficient removal rates of NPX [
82].
Table 3. Examples of biodegradation of pharmaceutical micropollutants by Lac coupled with reaction mediators.
Low molecular weight mediators allow complex compounds to access the active site of the enzyme. The oxidation of HBT by Lac generates small aminoxyl radicals that can remove the H atom from the O-H bond of phenolic substrates and consequently can create the phenoxyl radicals. The aforementioned mechanism may be responsible for the significant improvement in the degradation of salicylic acid [
86].
In contrast to the low efficiency of the Lac degradation process without mediators, Lac coupled with ABTS revealed complete degradation of tetracycline (TC) and oxytetracycline (OTC) after only 4 min and 5 min, respectively [
41]. Naghdi and co-authors [
56] observed that 95% removal of carbamazepine (CBZ) was possible due to the combination of ABTS with crude Lac from
T. versicolor. Furthermore, they reported that the enzyme concentration had a quadratic effect on biotransformation since an optimum level of Lac activity led to a rapid generation of ABTS radicals and caused an efficient transformation of CBZ, but further addition of enzyme to the solution increased the collisions and interactions and could block enzymes active sites. In the case of Lac-catalyzed degradation of the antibiotic chloramphenicol (CAP), Navada and co-authors [
51] concluded that natural mediators (SA and vanillin) exhibited lower K
m values than the reactions mediated by the synthetic mediators (ABTS and α-naphthol), showing that the natural mediators have a higher affinity to Lac than CAP and, therefore, increased the rate of oxidation reactions.
Ideally, during the oxidation of the pollutant, only oxygen is consumed in the catalytic cycle. However, the consumption of the mediator during the reaction is also possible. In this case, “Lac enhancer” is a more accurate term. Margot and co-authors [
81] investigated and showed the potential of the Lac-mediator system for the degradation of the antibiotic SMX with ABTS, SA and acetosyringone (AS). From their results, neither ABTS, SA nor AS acted as catalysts, since these three mediators were consumed during the reaction, with a mediator/pollutant molar ratio between 1.1 and 16. The authors proposed an alternative model of oxidation in which Lac oxidize mediators to reactive radicals, that can transform into more stable products and react with each other or with pollutants.
Bankole and co-authors [
84] demonstrated the effectiveness of ABTS, HBT and
p-coumaric acid as redox mediators in the degradation of olsalazine by Lac from
Aspergillus aculeatus. Furthermore, they observed that the increase in degradation efficiency was proportional to an increase in the concentration of mediators till a threshold value is reached, and at this point, no significant enhanced degradation of the pharmaceuticals occurs. This result is in line with the general trend observed in the literature that such threshold concentrations depend on the source of Lac, the target compound and the mediator used [
52].
On the other hand, Lac catalyzed reaction with TEMPO, which produces the oxoammonium cation, was able to greatly promote atenolol (ATL) transformation in an aqueous solution, with complete removal after 12 h [
85]. However, Wang et al. [
85] also found that Lac would be deactivated more rapidly in the reactions with higher mediator concentration as a result of distortion or blockage of enzyme active sites by radicals. Other findings show that free radicals produced by the oxidation of a mediator can destabilize the enzyme by reacting with the aromatic amino residues on its outer surfaces [
82].
Catalytic degradation using ligninolytic enzymes such as Lac with redox mediators may represent an alternative clean strategy for PhAC removal from the water matrix. However, the viability of this solution in real treatment systems is limited due to the necessity for high concentrations of mediator and the formation of several remediation products in concentrations eventually higher than the original pollutant [
81].
2.2. Transformation Mechanisms and Toxicity Evaluation
Enzymes transform complex compounds into simpler substances and it is unknown whether pharmaceuticals are metabolized by remediating enzymes to less or more toxic products. There is also a lack of knowledge on the structure of the metabolites resulting from pharmaceuticals degradation process. Therefore, some authors have studied the transformation pathways, as well as metabolites toxicity or estrogenic activity.
Considering molecular weight and chemical structure, Kózka et al. [
87] observed three main types of transformations of a series of antidepressants and immunosuppressants carried out by fungal ligninolytic enzymes. The first one is chemical oxidation and occurred for clomipramine, mianserin, sertraline, fluoxetine and citalopram. The second transformation is straight demethylation or demethylation coupled with other reactions such as oxidation or deamination, and was observed for clomipramine, mianserin, sertraline and venlafaxine transformation. The third type of transformation is the oxidative cleavage of the molecule into two parts of comparable size and was observed for fluoxetine and paroxetine.
Kasonga et al. [
88] proposed the metabolic pathways for ibuprofen (IBP) and CBZ based on detected intermediates by a fungal consortium of Lac, LiP and MnP. The IBP transformation pathway appeared to result from hydroxylation with addition of hydroxyl group to 1,2-dihydroxy-IBP or carboxylation reaction leading to the substitution of the methyl group by carboxylic group to form IBP carboxylic acid. Furthermore, the CBZ metabolic pathway was presented in four routes. The first route proposed was oxidation or hydrolysis to iminostilbene. The second route consisted of oxidation reactions of the carbons on the aromatic benzene group to CBZ-2,3-quinone. The third route combined hydroxylation, hydrolysis and then oxidization to iminoquinone. The fourth and principal metabolic route started with oxidation or epoxidation and the end products were acridone, 10,11-dihydro-10-hydroxy-CBZ and 9-hydroxymethyl-10-carbamoyl acridan. Likewise, according to Naghdi et al. [
56], 10,11-dihydro-10,11-dihydroxy-CBZ and 10,11-dihydro-10,11-epoxy-CBZ are considered to be the primary metabolites from CBZ oxidation by Lac-ABTS. Moreover, toxicity tests revealed that these products had no estrogenic effect.
In another study related to the degradation of DCF by Lac, the authors identified 3′-hydroxydiclofenac, 4′-hydroxydiclofenac, and 5-hydroxydiclofenac as the major transformation products [
54].
Yang et al. [
89] demonstrated that immobilized
C. unicolor Lac was effective in detoxification of TC antibiotics and identified three transformation products with LC-TOF-MS. According to the authors, TC is first oxidized by Lac to the corresponding ketone and then the amino group is bi-demethylated to form the second transformation product. The final product results from oxidation, followed by water elimination and dehydrogenation.
Lac oxidation of SMX in presence of ABTS possibly results in two products from pharmaceutical degradation and a third one from ABTS oxidation by Lac, designated 3-ethyl-6-sulfonate benzothiazolinone imine [
81]. In another study, Tian et al. [
41] demonstrated that TC was first oxidized by Lac-ABTS to OTC as the major transformation product and proposed a degradation pathway including deamination, demethylation and dehydration.
The assessment of the overall toxicity of the treated effluents is essential to provide a more complete outlook of the environmental relevance of the enzymatic treatments in real practical applications. Several metabolites result from enzymatic water treatment and additional toxicological information by means of bioassays is imperative. These studies are able to describe the ecotoxicity and the estrogenic activity of the resulting metabolites.
Presently, there are examples of pharmaceutical enzymatic degradation that produce fewer toxic compounds and lack of estrogenicity effect. A micro-toxicity study with
Pseudokirchneriella subcapitata,
Candida albicans,
Cryptococcus neoformans and
Saccharomyces cerevisiae revealed that Lac-HBT treated ketoconazole and its isolated metabolites, such as 1-(4-{4-[2-(2,4-dichloro-phenyl)-2-imidazol-1-ylmethyl-[1,3]dioxolan-4-ylmethoxy]-phenyl}-4-oxy-piperazin-1-yl)-ethanone, suffered a decrease in the toxicity levels. The presence of the oxygen atom in the structure of metabolites reduces their lipophilicity and decreases their toxicity [
83]. Furthermore, Lac-SA mediated system led to the transformation of CAP in chloramphenicol aldehyde and had less toxicity for microbial growth than mediators vanillin, ABTS and α-naphthol [
51].
Spina et al. [
90] showed that crude Lac from
Trametes pubescens MUT 2400 was very active against all target micropollutants as ketoprofen, present in real municipal wastewater. Estrogenic analysis and toxicological tests with
Raphidocelis subcapitata and
Lepidium sativum showed a clear ecotoxicity reduction of treated wastewater. Similarly, Sun et al. [
50] reported that a concentrated Lac isolated from
Trametes hirsuta was capable of effectively metabolize 17b-estradiol (E2) more than 99% and potentially lead to a reduction of the estrogenic activity of E2. Through the combination of 13C-isotope labelling with high-resolution mass spectrometry, the dimers, trimers and tetramers were recognized as the primary by-products of E2 metabolism.
Contrarily, Becker et al. [
91] verified that Lac mediated with SA effectively removes (>50%) a broad range of antibiotics after 24 h. However, this enhanced degradation induces unspecific toxicity. Furthermore, Feng et al. [
85] verified that the transformation of ATL via Lac/TEMPO-catalyzed reaction greatly reduced the mortalities of zebrafish (Danio rerio) eggs, but the degradation products and the residual TEMPO still possess toxicity (approximately 40%). This transformation mainly involved hydroxylation, carbonylation, C–O bond cleavage and coupling reactions.
2.3. Immobilized Biocatalytic System
The practical application of freely suspended enzymes exhibits high activity in the biotransformation processes. Nevertheless, the free form is limited by the low stability and high cost of production for large scale implementation due to the impossibility of recovery [
92,
93]. To overcome such limitations, several studies have already demonstrated that enzyme activity and stability can be improved by immobilization on solid supports [
36].
The stabilization of the peptide structure of the biocatalyst, creating interactions between the enzyme and an immobilization matrix, leads to enzyme stability and resistance improvement towards extreme operational conditions, including strong pH, high temperature or the presence of organic solvents [
94]. Furthermore, immobilization allows the easy recovery of the enzyme and offers high reusability in several catalytic cycles without significant loss of its unique properties, which reduces operating costs [
95].
The immobilization strategies are divided into methods based on physical or chemical interactions between enzymes and supports [
96]. Physical immobilization involves the creation of non-specific interactions via hydrogen bonds, ionic and hydrophobic interactions. The physical methods include entrapment, encapsulation and adsorption, and there is no requirement for the functionalization of the support [
36]. On the other hand, chemical immobilization includes enzyme attachment to the matrix by covalent binding or cross-linking [
97]. An example of a covalent binding agent is glutaraldehyde that is capable to react with the amine groups at the surface of both enzymes and support through the formation of Schiff’s bases and Michael’s adducts [
98]. Moreover, enzymes can be cross-linked to each other or create a cross-linked enzyme aggregate (CLEA) [
99].
Physical adsorption is simpler and leads to higher final enzyme activity. However, desorption or leakage of the immobilized enzyme is common with cycles of use due to the relatively weak binding forces. Oppositely, chemical immobilization leads to partial deformations in the enzyme molecular shape but offers a robust attachment of enzymes to the support [
94,
100]. The characteristics of the support are essential to define the success of the final biocatalyst, therefore the ideal support for usage of industrial applications should be inert, rigid, inexpensive, eco-friendly and present thermal and mechanical resistances [
24]. The support matrices can be classified according to their chemical composition as inorganic materials, organic materials, hybrids and composite materials. A large diversity of support has been developed due to the search for better stability and scale-up performance. Several recent methods of enzyme immobilization are summarized in
Table 4.
Table 4. Examples of the removal of pharmaceutical compounds by immobilized enzyme.
A wide range of materials are used for enzyme immobilization. Zdarta and co-authors [
93] studied Lac immobilization by adsorption and encapsulation using poly(l-lactic acid)-co-poly(ε-caprolactone) (PLCL) electrospun nanofibers. After 24 h, encapsulated Lac biodegraded over 90% of NPX and DFC, contrasting with an adsorbed enzyme which presented lower removal efficiency, 60% for NPX and 80% for DFC. This is mainly justified by the deactivation and elution of enzymes from the support. Dong and co-authors [
110] described a mediating system in which Lac was assembled, over π-π interactions, onto pristine few-layer graphene (FLG) surface. The composite effectively transformed beta-blocker labetalol for more than 10 cycles, as the FLG increases the exposure extent of the catalytic center with the enhancement of the catalytic activity.
Immobilization of enzymes provides protection against denaturation and conformational changes. For example, Sharifi-Bonab and co-authors [
23] immobilized Lac on graphene oxide (GO) nanosheets, followed by entrapment in alginate biopolymer. The immobilized Lac retained more than 70% of its initial activity after 10 days, contrarily to free Lac that became inactive. Furthermore, according to Zdarta and co-authors [
107], Lac immobilized in mesostructured cellular foam (MCF) silica retained higher activity (80%) over a wider range of temperature and pH. The enzyme immobilized onto MCF + Cu preserved almost 90% of its initial activity after 10 cycles since the MCF has a protective effect towards Lac molecules immobilized onto the surface and into its pores. Similarly, Lac can enter into the narrow mesopores of meso-MIL-53(Al) by undergoing conformational changes and becoming immobilized in the mesopore-MIL-53(Al), thus the entrapment force is significantly higher compared to conventional physical adsorption [
111]. In another example, Nguyen and co-authors [
96] immobilized Lac on acid-washed granular activated carbon (GAC) via physical adsorption and observed that GAC-bound Lac maintained full activity for up to 8 cycles of continuous application.
Covalent binding of enzymes on solid materials for pollutant removal has been intensively investigated and yields efficient results. Masjoudi and co-authors [
94] described a covalent immobilization of Lac on polyvinylidene fluoride (PVDF) membrane modified with multi-walled carbon nanotubes (MWCNT) and observed successfully removal of DCF (95% in 4 h) in a mini-membrane reactor. Likewise, Maryšková and co-authors [
105] immobilized the Lac onto polyamide/polyethylenimine (PA/PEI) nanofibers, via covalent attachment, with the Lac retaining more than 52% of initial activity after 30 days and successfully degrading triclosan (~70%) and 17α-ethynylestradiol (~50%) in real wastewater effluent. In another work, Lac immobilization on titania nanoparticles (TiO
2), with the functionalizing agent 3-aminopropyltriethoxysilane (APTES) and glutaraldehyde cross-linker, showed remarkable stability at 50 °C and 60 °C and low pH values of 2 and 3 [
101].
Compared with literature data, Apriceno and co-authors [
49] reported that a quite low value of enzymatic activity (0.02 U of enzyme) was required for 90% of degradation of DCF (50 mg/mL) in 3 h, when the Lac was covalently immobilized on chitosan beads and coupled with ABTS. Yaohua and co-authors [
108] also reported an efficient degradation of índole, even after 5 and 10 cycles, using a co-immobilization method, which consisted on the encapsulation of ABTS molecules into the dual-functionalized cellulose beads, followed by covalent binding of Lac.
Magnetic cross-linked enzyme aggregates (M-CLEA) are another example of the enzyme immobilization method, in which amino-functionalized magnetic nanoparticles are used. Yang and co-authors reported that Lac immobilized as M-CLEA eliminated over 80 µg/mL of TC in 12 h [
89]. Similarly, CLEA and M-CLEA Lac showed high DCF removal capacity (~80%) at 1 and 5 µg/L pollutant concentrations [
99].
The use of immobilized biocatalysts for PhAC transformation leads to sustainable industrial process performance. Despite the promising results, there are still issues to be further investigated such as the production costs of the immobilized enzymes, the possibility of scaling biocatalytic systems, as well as storage stability and the treatment potential of numerous PhAC in real wastewater effluents using enzyme immobilized systems.