Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vinayak Sharma | -- | 2468 | 2022-09-28 13:25:35 | | | |
| 2 | Rita Xu | Meta information modification | 2468 | 2022-09-29 02:59:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharma, V.; Javed, B.; Byrne, H.; Curtin, J.; Tian, F. Zeolites as Carriers of Nano-Fertilizers. Encyclopedia. Available online: https://encyclopedia.pub/entry/27900 (accessed on 07 February 2026).
Sharma V, Javed B, Byrne H, Curtin J, Tian F. Zeolites as Carriers of Nano-Fertilizers. Encyclopedia. Available at: https://encyclopedia.pub/entry/27900. Accessed February 07, 2026.
Sharma, Vinayak, Bilal Javed, Hugh Byrne, James Curtin, Furong Tian. "Zeolites as Carriers of Nano-Fertilizers" Encyclopedia, https://encyclopedia.pub/entry/27900 (accessed February 07, 2026).
Sharma, V., Javed, B., Byrne, H., Curtin, J., & Tian, F. (2022, September 28). Zeolites as Carriers of Nano-Fertilizers. In Encyclopedia. https://encyclopedia.pub/entry/27900
Sharma, Vinayak, et al. "Zeolites as Carriers of Nano-Fertilizers." Encyclopedia. Web. 28 September, 2022.
Copy Citation
The world is facing immense challenges in terms of food security, due to the combined impacts of the ever-increasing population and the adversity of climate change. In an attempt to counteract these factors, smart nutrient delivery systems, including nano-fertilizers, additives, and material coatings, have been introduced to increase food productivity to meet the growing food demand. Use of nanocarriers in agro-practices for sustainable farming contributes to achieving up to 75% nutrient delivery for a prolonged period to maintain nutrient availability in soil for plants in adverse soil conditions.
engineered nano-zeolites
controlled-release fertilizers
toxic effects
1. Introduction
The world’s food demand is rapidly growing due to a rapid increase in the global population, which is expected to rise to 9.6 billion by 2050. It is estimated that the annual grain production should increase by 70% to meet the food demand of the world’s population [1][2]. Over the past few decades, extensive use of agrochemicals has enhanced agricultural productivity, but also compromised human and soil health, disrupting food supplies due to the reduced agricultural yield [3]. At present, fertilizer contributes to improving 50% of total agriculture production, but increasing the dose of fertilizers does not always guarantee an increased crop production and yield. These agricultural practices demand excessive use of nutrients such as N, P, K, Ca, Mg, Fe, Zn, Mo, and B to enhance soil fertility and productivity, but might lead to soil contamination [4]. Despite the increased use of fertilizers, the rate of nutrient removal from the soil is much higher, resulting in a net-negative soil nutrient balance of about 10 million tons, and widespread economic loss to farmers [4]. Nutrient deficiency is a major contemporary problem. Nutrients contained in chemical fertilizers are not readily available to plants due to their macro size; thus, crops use as little as half of what is applied [5]. Furthermore, most macronutrients are insoluble in soil, and the unused fractions run off, contributing to the soil and water pollution. Overuse of chemical fertilizers has short-term gains in terms of an increase in crop yield or production, but poses long-term detrimental effects on the ecosystem.
Most developing and underdeveloped countries do not have proper legislation for using chemical fertilizers. In most cases, the chemical fertilizers are sprayed or drizzled onto plants without considering the nutritional conditions of the plant or soil. As a result of non-targeted strategies of conventional fertilizer application, the amounts of nutrients reaching the plant are much less than that lost through leaching and spillage from the agricultural fields to the water bodies and the soil. The other challenges of using conventional fertilizers include economic losses, environmental impacts including damaged microflora, disruption of ground food webs which leads to genetic mutations, changes in ecosystem ecology, reduced nitrogen fixation, and an increased number of pathogens and pests eventually affecting the soil flora and fauna [6].
The challenges of nutrient deficiency can potentially be addressed through nanotechnology-based solutions, and specifically targeted nutrient delivery through engineered nanoscale materials. Nanomaterials have the potential to revolutionize the agriculture sector by changing the food system, improving crop yield, preserving ecological balance, and fostering environmental sustainability [7]. With their small size, large surface area, high solubility, and mobility, these particles can be well dispersed in soil, and easily diffuse across plant cell membranes by soil or foliar treatment. Nanoparticles (NPs) can easily translocate in plants, promoting the release of nutrients through nano-fertilizers, and can also provide better protection using nano-pesticides and nano-herbicides.
A strong candidate to be employed as a carrier of nutrients are porous aluminosilicates known as zeolites. They are naturally occurring or can be synthesized chemically, whereby their porosity can be tuned on nanoscales, depending upon the application requirements. Naturally available zeolites include clinoptilolite, mordenite (MOR), erionite, phillipsite, analcime, lind type A (LTA), chabazite (CHA), beta-structured (BEA), sodalite (SOD), etc. They have different pore sizes, ion exchange properties, and bulk densities [8]. On the other hand, synthetic zeolites are prepared using different methodologies (template-assisted, template-free, hydrothermal treatment, etc.), with the advantages that their size, shape, and other physiochemical properties can be modified [9].
The unique properties of zeolites enable the prolonged and controlled introduction of necessary nutrients such as potassium, ammonium, and phosphates into the soil [10]. Studies have also shown that zeolites serve as slow-release sources of nutrients when they are pre-charged with ammonium–nitrogen (NH4 = N) and iron (Fe2+), then selected as components in soilless media [11]. In order to minimize nitrate leaching and volatilization, slow-release fertilizers with zeolites are needed to support the increasing demand of nitrogen in soil [12]. Both natural and synthetic zeolites are studied for their capability to load fertilizer and use as delivery molecules [13][14].
2. Smart Nutrient Delivery: Nano-Fertilizers and Their Mode of Action
Nano-fertilizers function as smart fertilizers which are either modified or synthesized from traditional fertilizers and bulk materials. They are of three different types:
- i.
-
Nanoscale fertilizers (nanoparticles of silica, iron, etc., which contain nutrients);
- ii.
-
Nanoscale additives (established fertilizers with nanoscale additives);
- iii.
-
Nanoscale coatings (fertilizers coated with nanoscale materials).
Nanotechnology in the agriculture sector is still in its infancy, as compared with medical and engineering applications. Nanoparticles have the potential to act as carriers of nutrients, leading to the concept of nanoscale fertilizers as smart nutrient delivery systems. Nano-fertilizers have advantages over routine agrochemical methods due to their large surface area, controlled release of nutrient formulations to match uptake patterns of the crops, increased nutrient uptake efficiency, solubility, and dispersion of micronutrient-directed release modes, and reduced loss rate of nutrients [6]. Nanocarriers have many advantages (Figure 1) because of their physicochemical properties, including their stability in media, their size, and biocompatibility, which helps to increase their shelf life in soil, resulting in efficient mixing of the fertilizer solution. Ordinary fertilizers, if used in excess, can have harmful effects on humans. However, the use of these delivery molecules helps to mitigate the drastic effects of ordinary fertilizers [15]. The encapsulation of fertilizers for the controlled release will not only protect the active ingredient, but will also affect the diffusion rate, the interactions of compounds with the environment, and their activity [16].
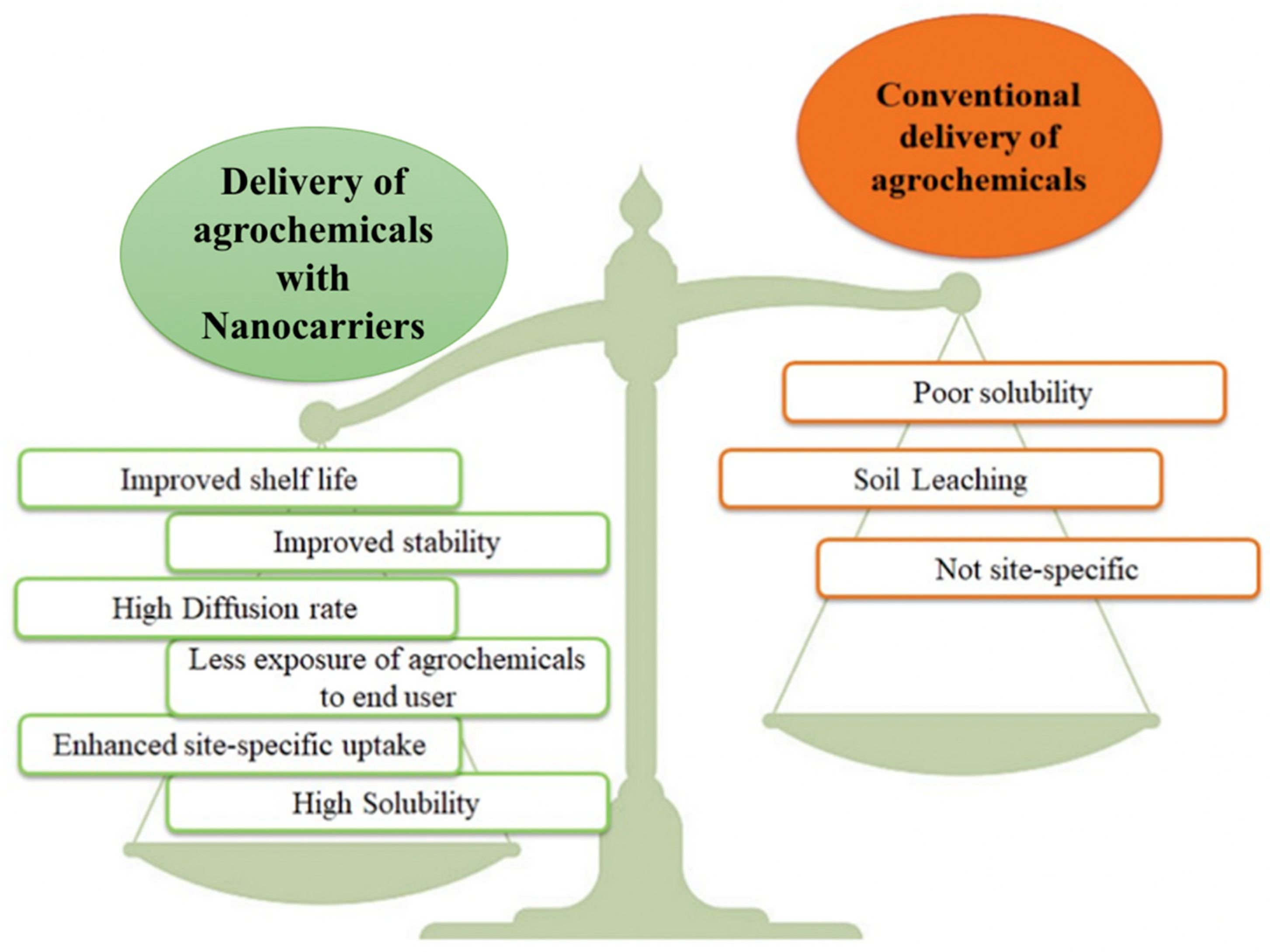
Figure 1. Advantages of delivery of agrochemicals using nanocarriers over conventional delivery.
Nanoscale fertilizers are smaller than the sizes of pores in the roots and leaves of plants, which increases their absorption and the uptake of nutrients in the plant body. Nano-zeolites can release nutrients to the plant body at a slow rate, which increases the availability of nutrients in crops and prevents the loss of nutrients from denitrification, volatilization, and leaching [17]. The surface coating of nanoparticles with various biocompatible polymers helps to slow the release of compounds; alternatively, the surface can be made more porous so that some of the nutrient content can be retained for a longer period. The nutrient release rate required by slow-release fertilizers (SRFs) is different, depending upon the requirements of crops. The European standardization committee task force has defined the criterion for SRFs that not more than 15% of nutrients are released within 24 h, with the remaining 75% within 28 days of application of the fertilizer [18].
The release of nutrients from nano-fertilizers is divided into three major stages: the lag period, linear period, and decay period [19][20]. In the lag stage, the water in the soil enters through the surface of the nano-fertilizers and slowly penetrates the core. During this stage, no fertilizer is released, and the vapor–pressure gradient created acts as the driving force. The lag is established to create a flux of water entering and a flux of solute leaving. In the second stage, the water level inside continues to increase, such that more fertilizer is dissolved, because of which osmotic pressure in the core builds, leading to slow release of the fertilizer through the pores [21]. The last stage is decay, during which the majority of the fertilizer has been released, reducing the concentration gradient, driving force, and increasing the release rate. The mechanism is described using a sigmoidal, S-shaped curve (Figure 2). If the internal pressure increases too rapidly in the lag phase, it may lead to a burst release of the nutrients, which is explained as a failure (dotted line); however, if the internal pressure increases linearly with time, then the nutrient molecules adsorbed in the pores will facilitate the slow-release mechanism depicted as the green sigmoidal curve. The prolonged release of nutrients from the source to the plants will promote growth, maintaining soil health.
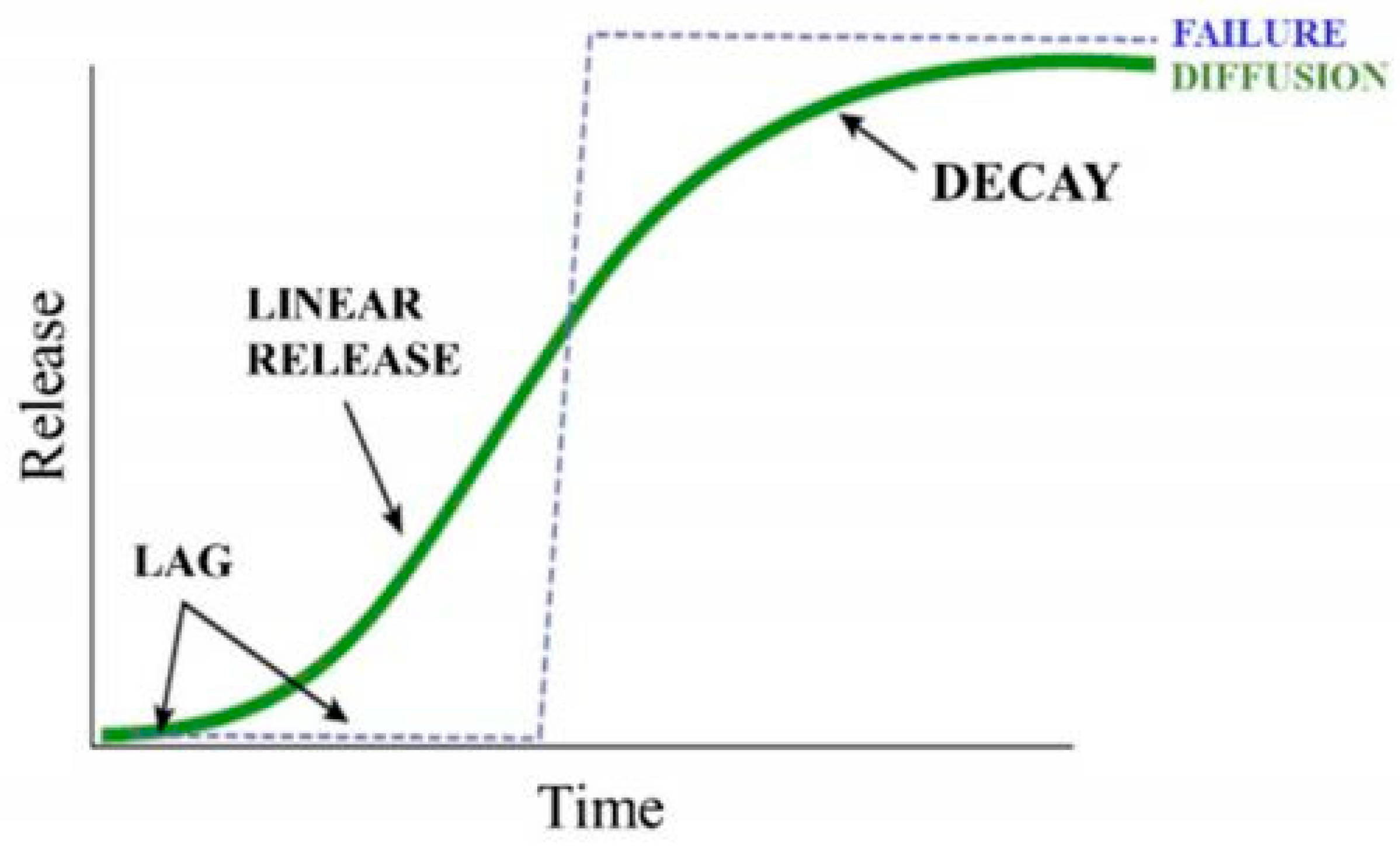
Figure 2. Sigmoidal curve for slow-release fertilizer (green) and failure release (blue). Reproduced from [22].
Nanoparticles such as zeolites have a high adsorption capacity to accumulate N, P, K, and S nutrients from precursor solutions due to their porous structure. They not only carry the nutrients, but also facilitate prolonged release as compared with bare nutrients provided to the plants. The enhanced micronutrient use efficiency with zeolite supplementation is also reported in many studies [18][22][23]. The improved availability of nutrients in the soil with the application of zeolites will ultimately facilitate enhanced nutrient availability to the plants.
3. Zeolites: Potential Candidates for Modern Agricultural Practices
Zeolites, often referred to as molecular sieves, are crystalline, hydrated alumina silicates of alkali and alkaline earth cations. They have a three-dimensional lattice which defines an inner network of interconnected pores and channels (Figure 3). The term ‘zeolite’ was coined by Swedish mineralogist Axel Fredrik Crønsted in 1756, who observed that, upon rapid heating, this material produced steam from water which it had adsorbed [24]. Based on this, he called the material “zeolite”, which originates from the Greek word ζέω (zéo̱), meaning “to boil” and λίθος (líthos), meaning “stone”. These molecular sieves are composed of TO4/2 tetrahedra, where T stands for Si, Al, Si, P, Ga, Ge, and B [24]. The basic structures of zeolites and their composing units are depicted in Figure 3. These materials have inbuilt channels and cages that crisscross the entire structure and make the crystalline framework accessible to foreign species. Appropriate hydrothermal conditions can lead to the crystallization of zeolites, linking Al (Si) tetrahedra (primary building blocks) into a corner-sharing network (secondary building network) through oxygen (O) atoms integrated by rings and prisms of different sizes. The combination of such units generates a structure with regular distribution of pores and cavities, with the pore size ranging from micropores (<2 nm), to mesopores (2–50 nm), to macropores (>50 nm). In a zeolite framework, Si and Al (being tetravalent and trivalent) give rise to SiO4 electro-neutral tetrahedra and AlO4 negatively charged tetrahedral, whereby this charge is compensated by extra cations provided by alkali and alkaline earth metals. The composition of a hydrated zeolite can be expressed by the formula:
where M is an extra framework cation with valence n, and x and y are the values of molar concentrations of Al and Si, respectively, in the zeolite structure. Z is the molar concentration of H2O [4]. Due to their unique physicochemical properties, zeolites have found a diverse range of applications, such as in water purification [25], chemical and radioactive waste remediation [26], catalysts in organic reactions [27], pesticide and herbicide management agents, etc. [28].
Mx/n, (H2O)z [(AlO2)x (SiO2)y]
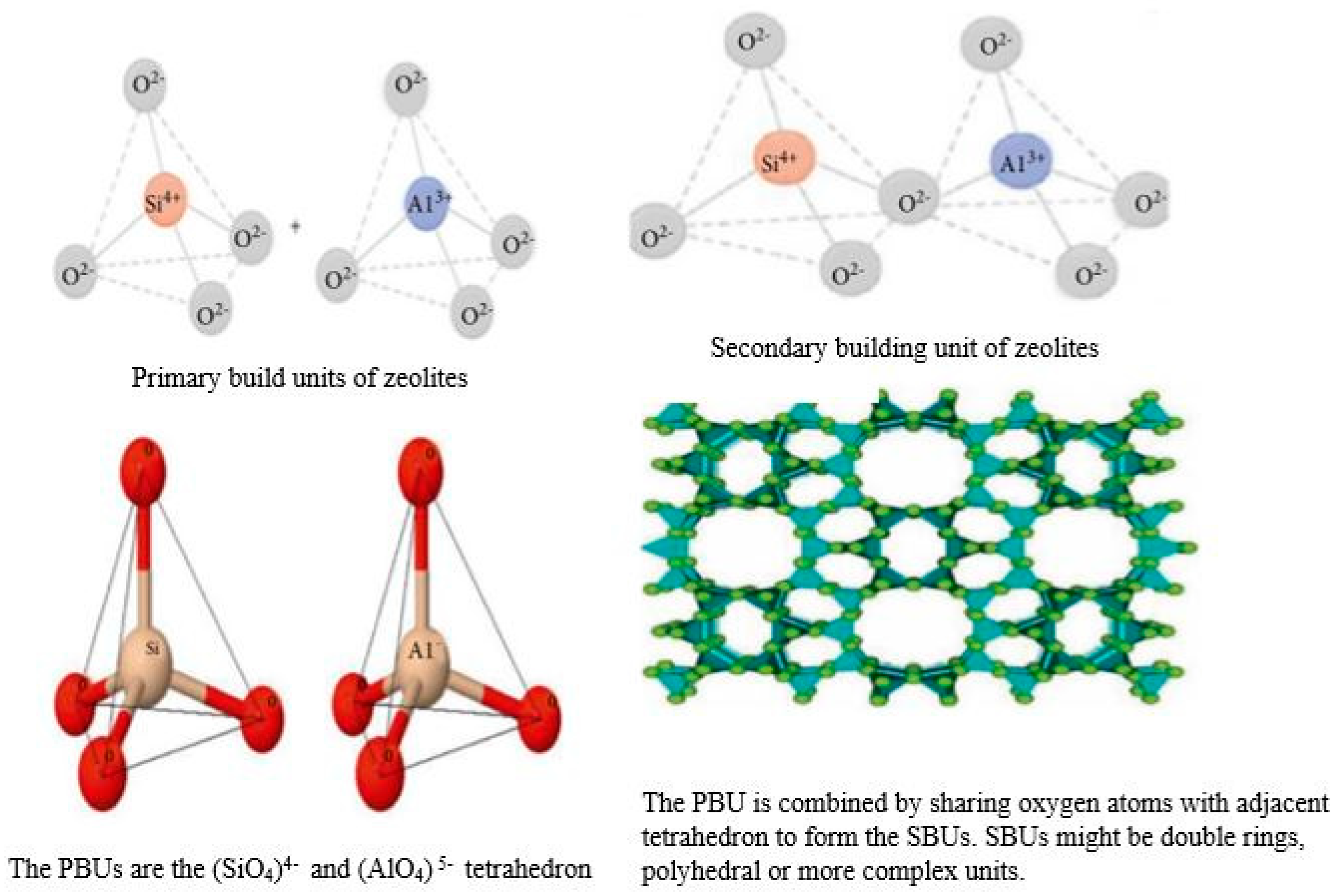
Figure 3. Structures of zeolite frameworks. Reproduced from [29].
Zeolites are mainly classified according to their silica/aluminium ratio into the following categories [23]:
-
Zeolite with a low Si-Al ratio (1–1.5): zeolite 4A, X, UZM-4 and UZM-5, etc.
-
Zeolite with intermediate Si-Al ratio (2–5): mordenite, LTA type, etc.
-
Zeolite with a high Si-Al ratio (10–several thousand): ZSM-5, ZSM-12, etc.
Zeolites also have a high void volume (50%), low mass density (2.1–2.2 g/cc) [25], high cation exchange capacity (CEC) of 150–250 cmol (+)/g, cation selectivity, for cations such as ammonium, potassium, cesium, etc. In a multi-component system, the selection of a zeolite depends upon various factors, including the hydration ratio, the exchangeable cation exchange capacity, the Si/Al ratio of zeolite, and the complementary ions as well as the temperature [29].
Zeolites are attractive materials due to their superior adsorption capacity and biocompatible nature [30]. Zeolites can have diverse applications, including:
These materials can be used as carriers, with optimized physicochemical and biological properties, which can permeate cell membranes more easily than large molecules; thus, they can be used as delivery tools of bioactive compounds [40]. The potential usage of zeolites for drug release [41] and biomedical applications can be improved by providing proper support to zeolite particles, to secure adhesion to tissues [42] and tailor drug release kinetics [43]. Due to their documented adsorption and ion exchange properties, zeolites can potentially be used for the cost-effective removal of pollutants from water, air, and soil [19]. Comparing natural and synthetic zeolites, the latter are preferred due to their enhanced texture parameters and diverse physicochemical properties [44]. Among natural zeolites, clinoptilolite is commonly used in agriculture and environmental applications, and its sorption and exchange properties can be modified by physical and chemical treatments [45][46]. However, the full potential of zeolites to remove toxic and harmful contaminants has not been explored to date [47].
Fertilizers play a significant role in agriculture; however, to further enhance the efficiency of nutrient use and control the long-term problem of eutrophication, nano-fertilizers have been identified as a potential route for significant development [48]. In this context, zeolites have been employed as carriers for the slow and targeted release of essential macro- and micronutrients to plants [49]. They reduce nitrogen loss due to leaching and allow selective release linked to time or environmental conditions. Nano porous zeolites have also been used as carriers in pest management, herbicide delivery, and as sensors in pest detection [50][51][52]. Improvement in soil physical properties, by reducing the bulk density, which, in turn, improves the water holding capacity and soil air porosity, is a benefit of applying zeolites in agricultural applications [35]. Zeolites are also considered water reservoirs, retaining soil moisture for a longer time during dry periods and enabling plants to survive during such conditions. It has been reported that soil amelioration with zeolites increases the water availability to plants by 50% [52]. This property was exploited by Moritani et al. [53], such that the incorporation of 10% of artificial zeolites in sodic soil resulted in improved wet aggregate stability. Different types of natural zeolites (mordenite, clinoptilolite, and stilbite) and synthetic zeolites have demonstrated positive changes in the soil properties, when applied to various textural classes of soil (sandy, loamy, clay, etc.), including water content, infiltration rate and hydraulic conductivity [54].
Increasing the use of zeolites leads to more demand for feasible methods for the development of hierarchical porous zeolites. Depending upon the desired properties, such as zeolite size, ordered channels, and defined pore diameter, the synthesis methods can be tuned [55]. Some methods employed for the fabrication of zeolites with enhanced physicochemical properties are discussed in the forthcoming section.
References
- United Nations. World Population Prospects: The 2012 Revision. Population Division of the Department of Economic and Social Affairs. 2013. Available online: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:World+Population+Prospects+The+2012+Revision#1 (accessed on 1 July 2022).
- Connor, D.J.; Loomis, R.S.; Cassman, K.G. Connor, Crop Ecology: Productivity and Management in Agricultural Systems; Cambridge University Press: Cambridge, UK, 2011.
- Prashar, P.; Shah, S. Impact of Fertilizers and Pesticides on Soil Microflora in Agriculture. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2016; pp. 331–361.
- Rai, M.; Ribeiro, C.; Mattoso, L.; Duran, N. (Eds.) Nanotechnologies in Food and Agriculture; Springer: Berlin, Germany, 2015; 347p.
- Chugh, G.; Siddique, K.; Solaiman, Z. Nanobiotechnology for Agriculture: Smart Technology for Combating Nutrient Deficiencies with Nanotoxicity Challenges. Sustainability 2021, 13, 1781.
- Acharya, A.; Pal, P.K. Agriculture nanotechnology: Translating research outcome to field applications by influencing environmental sustainability. NanoImpact 2020, 19, 100232.
- Tzia, C.; Zorpas, A.A. Zeolites in Food Processing Industries. In Handbook of Natural Zeolites; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 601–651.
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622.
- Bernardi, A.C.D.C.; Polidoro, J.C.; Monte, M.B.D.M.; Pereira, E.I.; de Oliveira, C.R.; Ramesh, K. Enhancing Nutrient Use Efficiency Using Zeolites Minerals—A Review. Adv. Chem. Eng. Sci. 2016, 06, 295–304.
- Williams, K.A.; Nelson, P.V. Using Precharged Zeolite as a Source of Potassium and Phosphate in a Soilless Container Medium during Potted Chrysanthemum Production. J. Am. Soc. Hortic. Sci. 1997, 122, 703–708.
- Aghaalikhani, M.; Gholamhoseini, M.; Dolatabadian, A.; Khodaei-Joghan, A.; Asilan, K.S. Zeolite influences on nitrate leaching, nitrogen-use efficiency, yield and yield components of canola in sandy soil. Arch. Agron. Soil Sci. 2012, 58, 1149–1169.
- Li, Z.; Zhang, Y. Use of surfactant-modified zeolite to carry and slowly release sulfate. Desalin. Water Treat. 2010, 21, 73–78.
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189.
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers—A review. Catena 2022, 213, 106125.
- Yuvaraj, M.; Subramanian, K.S. Novel Slow Release Nanocomposite Fertilizers. In Nanotechnology and the Environment; IntechOpen: Vienna, Austria, 2020.
- Elizabath, A.; Rme, S.I. Application of Nanotechnology in Agriculture. Int. J. Pure Appl. Biosci. 2019, 7, 131–139.
- Sheta, A.; Falatah, A.; Al-Sewailem, M.; Khaled, E.; Sallam, A. Sorption characteristics of zinc and iron by natural zeolite and bentonite. Microporous Mesoporous Mater. 2003, 61, 127–136.
- Shaviv, A.; Raban, S.; Zaidel, E. Modeling Controlled Nutrient Release from Polymer Coated Fertilizers: Diffusion Release from Single Granules. Environ. Sci. Technol. 2003, 37, 2251–2256.
- Sempeho, S.I.; Kim, H.T.; Mubofu, E.; Hilonga, A. Meticulous Overview on the Controlled Release Fertilizers. Adv. Chem. 2014, 2014, 363071.
- Irfan, S.A.; Razali, R.; KuShaari, K.; Mansor, N.; Azeem, B.; Versypt, A.N.F. A review of mathematical modeling and simulation of controlled-release fertilizers. J. Control. Release 2018, 271, 45–54.
- Trenkel, M.E. Slow-and Controlled-Release and Stabilized Fertilizers: An Option for Enhancing Nutrient Use Effiiency in Agriculture; IFA (International Fertilizer Industry Association): Paris, France, 2010.
- Iskander, A.; Khald, E.; Sheta, A.E.-A. Zinc and manganese sorption behavior by natural zeolite and bentonite. Ann. Agric. Sci. 2011, 56, 43–48.
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989.
- Mintova, S. Nanosized Molecular Sieves. Collect. Czechoslov. Chem. Commun. 2003, 68, 2032–2054.
- Kalló, D. Applications of Natural Zeolites in Water and Wastewater Treatment. Rev. Miner. Geochem. 2001, 45, 519–550.
- Jiménez-Reyes, M.; Almazán-Sánchez, P.; Solache-Ríos, M. Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 2021, 233, 106610.
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502.
- Sangeetha, C.; Baskar, P. Zeolite and its potential uses in agriculture: A critical review. Agric. Rev. 2016, 37.
- Derbe, T.; Temesgen, S.; Bitew, M. A Short Review on Synthesis, Characterization, and Applications of Zeolites. Adv. Mater. Sci. Eng. 2021, 2021, 6637898.
- Bhattacharyya, T.; Chandran, P.; Ray, S.K.; Pal, D.K.; Mandal, C.; Mandal, D.K. Distribution of Zeolitic Soils in India. Curr. Sci. 2015, 109, 1305.
- Sekhon, B.S.; Sangha, M.K. Detergents—Zeolites and enzymes excel cleaning power. Resonance 2004, 9, 35–45.
- Li, R.; Chong, S.; Altaf, N.; Gao, Y.; Louis, B.; Wang, Q. Synthesis of ZSM-5/Siliceous Zeolite Composites for Improvement of Hydrophobic Adsorption of Volatile Organic Compounds. Front. Chem. 2019, 7, 505.
- Ming, D.W.; Allen, E.R. Use of Natural Zeolites in Agronomy, Horticulture and Environmental Soil Remediation. Rev. Miner. Geochem. 2001, 45, 619–654.
- Ramesh, K.; Reddy, D.D. Zeolites and Their Potential Uses in Agriculture. Adv. Agron. 2011, 113, 219–241.
- Leggo, P.J. An investigation of plant growth in an organo-zeolitic substrate and its ecological significance. Plant Soil 2000, 219, 135–146.
- Valente, S.; Burriesci, N.; Cavallaro, S.; Galvagno, S.; Zipelli, C. Utilization of zeolites as soil conditioner in tomato-growing. Zeolites 1982, 2, 271–274.
- Campbell, L.S.; Davies, B.E. Experimental Investigation of Plant Uptake of Caesium from Soils Amended with Clinoptilolite and Calcium Carbonate; Springer: Berlin, Germany, 2021; Volume 189, pp. 65–74. Available online: https://www.jstor.org/stable/42947951 (accessed on 1 July 2022).
- Al Dwairi, R.A.; Aiman, A. Recent Patents of Natural Zeolites Applications in Environment, Agriculture and Pharmaceutical Industry. Recent Patents Chem. Eng. 2012, 5, 20–27.
- Sun, C.-Y.; Qin, C.; Wang, X.-L.; Yang, G.-S.; Shao, K.-Z.; Lan, Y.-Q.; Su, Z.-M.; Huang, P.; Wang, C.-G.; Wang, E.-B. Zeolitic imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2012, 41, 6906–6909.
- Mahmodi, G.; Zarintaj, P.; Taghizadeh, A.; Taghizadeh, M.; Manouchehri, S.; Dangwal, S.; Ronte, A.; Ganjali, M.R.; Ramsey, J.D.; Kim, S.-J.; et al. From microporous to mesoporous mineral frameworks: An alliance between zeolite and chitosan. Carbohydr. Res. 2020, 489, 107930.
- Serati-Nouri, H.; Jafari, A.; Roshangar, L.; Dadashpour, M.; Pilehvar-Soltanahmadi, Y.; Zarghami, N. Biomedical applications of zeolite-based materials: A review. Mater. Sci. Eng. C 2020, 116, 111225.
- Rahmani, S.; Azizi, S.N.; Asemi, N. Application of Synthetic Nanozeolite Sodalite in Drug Delivery. Int. Curr. Pharm. J. 2016, 5, 55–58. Available online: http://www.icpjonline.com/documents/Vol5Issue6/02.pdf (accessed on 1 July 2022).
- Sotoudeh, S.; Barati, A.; Davarnejad, R.; Farahani, M.A. Antibiotic release process from hydrogel nano zeolite composites. Middle East J. Sci. Res. 2012, 12, 392–396.
- Qotob, M.A.; Nasef, M.A.; Elhakim, H.K.; Shaker, O.G.; Abdelhamid, I.A. Revisiting of chemical fertilizers by using suitable plant growth regulators and nano fertilizer. GSJ 2020, 8, 1896–1907.
- Khaleque, A.; Alam, M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir, M.; Karmakar, A.K.; Zhou, J.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019.
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Hazard. Mater. 2020, 393, 122481.
- Cieśla, J.; Franus, W.; Franus, M.; Kedziora, K.; Gluszczyk, J.; Szerement, J.; Jozefaciuk, G. Environmental-Friendly Modifications of Zeolite to Increase Its Sorption and Anion Exchange Properties, Physicochemical Studies of the Modified Materials. Materials 2019, 12, 3213.
- Ren, H.; Jiang, J.; Wu, D.; Gao, Z.; Sun, Y.; Luo, C. Selective Adsorption of Pb(II) and Cr(VI) by Surfactant-Modified and Unmodified Natural Zeolites: A Comparative Study on Kinetics, Equilibrium, and Mechanism. Water Air Soil Pollut. 2016, 227, 101.
- Qureshi, A.; Singh, D.; Dwivedi, S. Nano-fertilizers: A Novel Way for Enhancing Nutrient Use Efficiency and Crop Productivity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3325–3335.
- Mahabadi, A.A.; Hajabbasi, M.; Khademi, H.; Kazemian, H. Soil cadmium stabilization using an Iranian natural zeolite. Geoderma 2007, 137, 388–393.
- Bernardi, A.C.D.C.; Oliviera, P.P.A.; Monte, M.B.D.M.; Souza-Barros, F. Brazilian sedimentary zeolite use in agriculture. Microporous Mesoporous Mater. 2013, 167, 16–21.
- Groen, J.C.; Jansen, J.C.; Moulijn, A.J.A.; Pérez-Ramírez, J. Optimal Aluminum-Assisted Mesoporosity Development in MFI Zeolites by Desilication. J. Phys. Chem. B 2004, 108, 13062–13065.
- Moritani, S.; Yamamoto, T.; Andry, H.; Inoue, M.; Yuya, A.; Kaneuchi, T. Effectiveness of artificial zeolite amendment in improving the physicochemical properties of saline-sodic soils characterised by different clay mineralogies. Soil Res. 2010, 48, 470–479.
- Gholizadeh-Sarabi, S.; Sepaskhah, A.R. Effect of zeolite and saline water application on saturated hydraulic conductivity and infiltration in different soil textures. Arch. Agron. Soil Sci. 2013, 59, 753–764.
- Mintova, S.; Gilson, J.-P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693–6703.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
29 Sep 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




