Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Vinayak Sharma and Version 2 by Rita Xu.
The world is facing immense challenges in terms of food security, due to the combined impacts of the ever-increasing population and the adversity of climate change. In an attempt to counteract these factors, smart nutrient delivery systems, including nano-fertilizers, additives, and material coatings, have been introduced to increase food productivity to meet the growing food demand. Use of nanocarriers in agro-practices for sustainable farming contributes to achieving up to 75% nutrient delivery for a prolonged period to maintain nutrient availability in soil for plants in adverse soil conditions.
- engineered nano-zeolites
- controlled-release fertilizers
- toxic effects
1. Introduction
The world’s food demand is rapidly growing due to a rapid increase in the global population, which is expected to rise to 9.6 billion by 2050. It is estimated that the annual grain production should increase by 70% to meet the food demand of the world’s population [1][2][1,2]. Over the past few decades, extensive use of agrochemicals has enhanced agricultural productivity, but also compromised human and soil health, disrupting food supplies due to the reduced agricultural yield [3]. At present, fertilizer contributes to improving 50% of total agriculture production, but increasing the dose of fertilizers does not always guarantee an increased crop production and yield. These agricultural practices demand excessive use of nutrients such as N, P, K, Ca, Mg, Fe, Zn, Mo, and B to enhance soil fertility and productivity, but might lead to soil contamination [4]. Despite the increased use of fertilizers, the rate of nutrient removal from the soil is much higher, resulting in a net-negative soil nutrient balance of about 10 million tons, and widespread economic loss to farmers [4]. Nutrient deficiency is a major contemporary problem. Nutrients contained in chemical fertilizers are not readily available to plants due to their macro size; thus, crops use as little as half of what is applied [5]. Furthermore, most macronutrients are insoluble in soil, and the unused fractions run off, contributing to the soil and water pollution. Overuse of chemical fertilizers has short-term gains in terms of an increase in crop yield or production, but poses long-term detrimental effects on the ecosystem.
Most developing and underdeveloped countries do not have proper legislation for using chemical fertilizers. In most cases, the chemical fertilizers are sprayed or drizzled onto plants without considering the nutritional conditions of the plant or soil. As a result of non-targeted strategies of conventional fertilizer application, the amounts of nutrients reaching the plant are much less than that lost through leaching and spillage from the agricultural fields to the water bodies and the soil. The other challenges of using conventional fertilizers include economic losses, environmental impacts including damaged microflora, disruption of ground food webs which leads to genetic mutations, changes in ecosystem ecology, reduced nitrogen fixation, and an increased number of pathogens and pests eventually affecting the soil flora and fauna [6].
The challenges of nutrient deficiency can potentially be addressed through nanotechnology-based solutions, and specifically targeted nutrient delivery through engineered nanoscale materials. Nanomaterials have the potential to revolutionize the agriculture sector by changing the food system, improving crop yield, preserving ecological balance, and fostering environmental sustainability [7]. With their small size, large surface area, high solubility, and mobility, these particles can be well dispersed in soil, and easily diffuse across plant cell membranes by soil or foliar treatment. Nanoparticles (NPs) can easily translocate in plants, promoting the release of nutrients through nano-fertilizers, and can also provide better protection using nano-pesticides and nano-herbicides.
A strong candidate to be employed as a carrier of nutrients are porous aluminosilicates known as zeolites. They are naturally occurring or can be synthesized chemically, whereby their porosity can be tuned on nanoscales, depending upon the application requirements. Naturally available zeolites include clinoptilolite, mordenite (MOR), erionite, phillipsite, analcime, lind type A (LTA), chabazite (CHA), beta-structured (BEA), sodalite (SOD), etc. They have different pore sizes, ion exchange properties, and bulk densities [8]. On the other hand, synthetic zeolites are prepared using different methodologies (template-assisted, template-free, hydrothermal treatment, etc.), with the advantages that their size, shape, and other physiochemical properties can be modified [9].
The unique properties of zeolites enable the prolonged and controlled introduction of necessary nutrients such as potassium, ammonium, and phosphates into the soil [10]. Studies have also shown that zeolites serve as slow-release sources of nutrients when they are pre-charged with ammonium–nitrogen (NH4 = N) and iron (Fe2+), then selected as components in soilless media [11]. In order to minimize nitrate leaching and volatilization, slow-release fertilizers with zeolites are needed to support the increasing demand of nitrogen in soil [12]. Both natural and synthetic zeolites are studied for their capability to load fertilizer and use as delivery molecules [13][14][13,14].
2. Smart Nutrient Delivery: Nano-Fertilizers and Their Mode of Action
Nano-fertilizers function as smart fertilizers which are either modified or synthesized from traditional fertilizers and bulk materials. They are of three different types:- i.
-
Nanoscale fertilizers (nanoparticles of silica, iron, etc., which contain nutrients);
- ii.
-
Nanoscale additives (established fertilizers with nanoscale additives);
- iii.
-
Nanoscale coatings (fertilizers coated with nanoscale materials).Nanoscale fertilizers are smaller than the sizes of pores in the roots and leaves of plants, which increases their absorption and the uptake of nutrients in the plant body. Nano-zeolites can release nutrients to the plant body at a slow rate, which increases the availability of nutrients in crops and prevents the loss of nutrients from denitrification, volatilization, and leaching [17][21]. The surface coating of nanoparticles with various biocompatible polymers helps to slow the release of compounds; alternatively, the surface can be made more porous so that some of the nutrient content can be retained for a longer period. The nutrient release rate required by slow-release fertilizers (SRFs) is different, depending upon the requirements of crops. The European standardization committee task force has defined the criterion for SRFs that not more than 15% of nutrients are released within 24 h, with the remaining 75% within 28 days of application of the fertilizer [18][22]. The release of nutrients from nano-fertilizers is divided into three major stages: the lag period, linear period, and decay period [19][20][23,24]. In the lag stage, the water in the soil enters through the surface of the nano-fertilizers and slowly penetrates the core. During this stage, no fertilizer is released, and the vapor–pressure gradient created acts as the driving force. The lag is established to create a flux of water entering and a flux of solute leaving. In the second stage, the water level inside continues to increase, such that more fertilizer is dissolved, because of which osmotic pressure in the core builds, leading to slow release of the fertilizer through the pores [21][25]. The last stage is decay, during which the majority of the fertilizer has been released, reducing the concentration gradient, driving force, and increasing the release rate. The mechanism is described using a sigmoidal, S-shaped curve (Figure 2). If the internal pressure increases too rapidly in the lag phase, it may lead to a burst release of the nutrients, which is explained as a failure (dotted line); however, if the internal pressure increases linearly with time, then the nutrient molecules adsorbed in the pores will facilitate the slow-release mechanism depicted as the green sigmoidal curve. The prolonged release of nutrients from the source to the plants will promote growth, maintaining soil health.
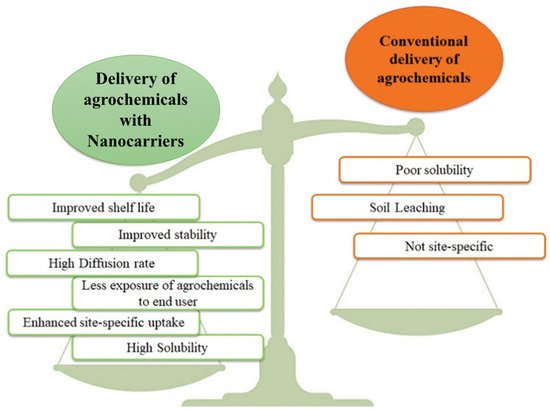 Figure 1. Advantages of delivery of agrochemicals using nanocarriers over conventional delivery.Nanoparticles such as zeolites have a high adsorption capacity to accumulate N, P, K, and S nutrients from precursor solutions due to their porous structure. They not only carry the nutrients, but also facilitate prolonged release as compared with bare nutrients provided to the plants. The enhanced micronutrient use efficiency with zeolite supplementation is also reported in many studies [18][22][23][22,26,27]. The improved availability of nutrients in the soil with the application of zeolites will ultimately facilitate enhanced nutrient availability to the plants.
Figure 1. Advantages of delivery of agrochemicals using nanocarriers over conventional delivery.Nanoparticles such as zeolites have a high adsorption capacity to accumulate N, P, K, and S nutrients from precursor solutions due to their porous structure. They not only carry the nutrients, but also facilitate prolonged release as compared with bare nutrients provided to the plants. The enhanced micronutrient use efficiency with zeolite supplementation is also reported in many studies [18][22][23][22,26,27]. The improved availability of nutrients in the soil with the application of zeolites will ultimately facilitate enhanced nutrient availability to the plants.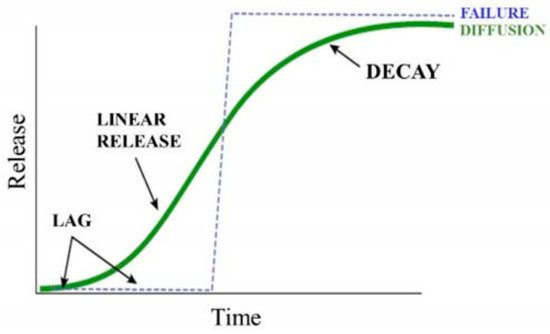
3. Zeolites: Potential Candidates for Modern Agricultural Practices
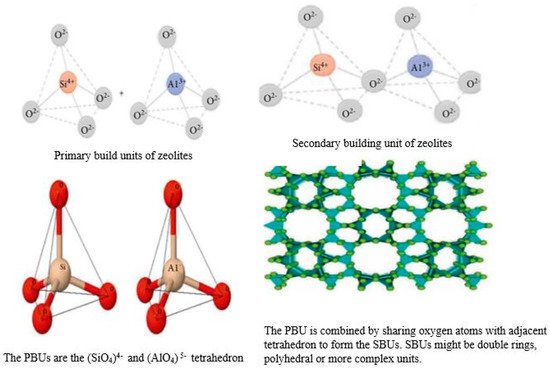
Zeolites, often referred to as molecular sieves, are crystalline, hydrated alumina silicates of alkali and alkaline earth cations. They have a three-dimensional lattice which defines an inner network of interconnected pores and channels (Figure 3). The term ‘zeolite’ was coined by Swedish mineralogist Axel Fredrik Crønsted in 1756, who observed that, upon rapid heating, this material produced steam from water which it had adsorbed [24][28]. Based on this, he called the material “zeolite”, which originates from the Greek word ζέω (zéo̱), meaning “to boil” and λίθος (líthos), meaning “stone”. These molecular sieves are composed of TO4/2 tetrahedra, where T stands for Si, Al, Si, P, Ga, Ge, and B [24][28]. The basic structures of zeolites and their composing units are depicted in Figure 3. These materials have inbuilt channels and cages that crisscross the entire structure and make the crystalline framework accessible to foreign species. Appropriate hydrothermal conditions can lead to the crystallization of zeolites, linking Al (Si) tetrahedra (primary building blocks) into a corner-sharing network (secondary building network) through oxygen (O) atoms integrated by rings and prisms of different sizes. The combination of such units generates a structure with regular distribution of pores and cavities, with the pore size ranging from micropores (<2 nm), to mesopores (2–50 nm), to macropores (>50 nm). In a zeolite framework, Si and Al (being tetravalent and trivalent) give rise to SiO4 electro-neutral tetrahedra and AlO4 negatively charged tetrahedral, whereby this charge is compensated by extra cations provided by alkali and alkaline earth metals. The composition of a hydrated zeolite can be expressed by the formula:where M is an extra framework cation with valence n, and x and y are the values of molar concentrations of Al and Si, respectively, in the zeolite structure. Z is the molar concentration of H2O [4]. Due to their unique physicochemical properties, zeolites have found a diverse range of applications, such as in water purification [25][29], chemical and radioactive waste remediation [26][30], catalysts in organic reactions [27][31], pesticide and herbicide management agents, etc. [28][32]. Zeolites are mainly classified according to their silica/aluminium ratio into the following categories [23][27]:Mx/n, (H2O)z [(AlO2)x (SiO2)y]-
Zeolite with a low Si-Al ratio (1–1.5): zeolite 4A, X, UZM-4 and UZM-5, etc.
-
Zeolite with intermediate Si-Al ratio (2–5): mordenite, LTA type, etc.
-
Zeolite with a high Si-Al ratio (10–several thousand): ZSM-5, ZSM-12, etc.
-
Aquaculture: filtering ammonia in fish hatcheries and as biofilter media.