Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianping Wu | -- | 1418 | 2022-09-26 11:11:45 | | | |
| 2 | Dean Liu | Meta information modification | 1418 | 2022-09-28 03:59:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, J.; Zahra, A.; Wang, Y.; Wu, J. Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter. Encyclopedia. Available online: https://encyclopedia.pub/entry/27585 (accessed on 07 February 2026).
Wang J, Zahra A, Wang Y, Wu J. Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter. Encyclopedia. Available at: https://encyclopedia.pub/entry/27585. Accessed February 07, 2026.
Wang, Jingjing, Aqeela Zahra, Yunfu Wang, Jianping Wu. "Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter" Encyclopedia, https://encyclopedia.pub/entry/27585 (accessed February 07, 2026).
Wang, J., Zahra, A., Wang, Y., & Wu, J. (2022, September 26). Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter. In Encyclopedia. https://encyclopedia.pub/entry/27585
Wang, Jingjing, et al. "Physiological Role of Electroneutral Na+-Coupled HCO3− Cotransporter." Encyclopedia. Web. 26 September, 2022.
Copy Citation
Acid–base homeostasis is critical for proper physiological function and pathology. The SLC4 family of HCO3− transmembrane cotransporters is one of the HCO3− transmembrane transport carriers responsible for cellular pH regulation and the uptake or secretion of HCO3− in epithelial cells. NBCn1 (SLC4A7), an electroneutral Na+/HCO3− cotransporter, is extensively expressed in several tissues and functions as a cotransporter for net acid extrusion after cellular acidification.
Na+/HCO3− cotransporter
central nervous system
pH
cardiovascular system
1. Introduction
The maintenance of intracellular pH (pHi) and extracellular pH (pHo) is critical for biological function. The fluctuations in pH affect the enzymatic activity, as well as the function of the cell membrane and the signaling molecules, which inevitably contribute to modifications in cellular activity [1]. The Na+/H+ exchangers and the Na+-dependent/independent HCO3− transporters are the primary transporters that are responsible for mediating net acid extrusion in the majority of mammalian cells. HCO3− is one of the most essential acid–base buffer ions and is involved in regulating the pH of two gene families in vertebrates, invertebrates, and humans (SLC4 and SLC26) [2].
SLC4 consists of 10 genes, nine of which encode HCO3− cotransporters that are either Na+-independent (AEs) or Na+-dependent (NBCs) [3][4]. Until now, SLC4 family members are the only molecular entities functionally shown as Na+-dependent HCO3− transporters. The Na+-dependent HCO3− transporters are further divided into electrogenic cotransporters NBCe1 (SLC4A4) and NBCe2 (SLC4A5), electroneutral cotransporters NBCn1(SLC4A7) and NBCn2(SLC4A10), electrically neutral Na+-driven Cl−/HCO3− exchanger (NDCBE) [5][6]. Despite their obvious uniformity of more than 30% amino acid sequence identities between these proteins, NBCs vary in three major aspects, for example, ion selectivity, transportation pattern, and cellular localization.
The NBCs family is essential in the regulation of pH and the transdermal release and uptake of Na+, HCO3−, and Cl− in different tissues [7][8]. The mode of transport, along with membrane potential and ion gradient, is a key factor in determining the direction of transport. At resting membrane potential and normal ion gradient, HCO3− and Na+ are transported 1:1 or 2:1 into the cell and transported 3:1 out of the cell. In some pathophysiological conditions, such as ischemia, cancer, etc., the transmembrane ion gradient and membrane potential change significantly, which may affect the flow of transport as well as the activity of acid–base cotransporters. Within the cytosol and interstitial space near the transport site, the transmembrane transfer of HCO3− occurs in the chemical reaction CO2+H2O⇔HCO3−+H+, which is catalyzed by carbonic anhydrase [9]. When equilibrium is reached, the transport of HCO3− is equivalent to the transport of H+ in the opposite direction, as shown in Figure 1. NBCn1 transports Na+ and HCO3− with a stoichiometry of 1:1, whereas NBCe1 and NBCe2 can transport with a stoichiometry of 1:2 or 1:3, depending on the cell type and phosphorylation status [10]. NCDBE facilitates electroneutral Na+-dependent Cl−/HCO3− exchange. It remains controversial whether the Na+-dependent HCO3− transport mediated by NCBE/NBCn2 is coupled to net Cl− transport or whether it is solely related to Cl− self-exchange.
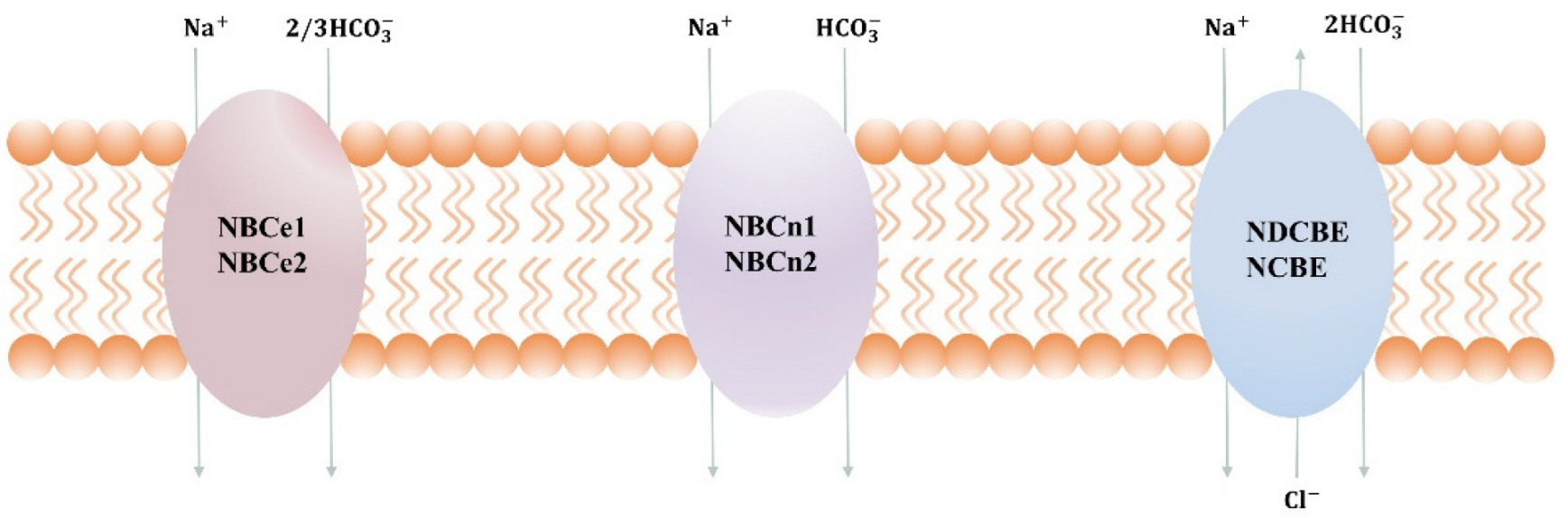
Figure 1. Transport stoichiometry of NBCs at intracellular (I) and extracellular (o) ion concentrations and membrane potentials. Note that NBCn2 and NCBE are the two alternative names suggested for SLC4A10, depending on whether the transporter mediates net Cl− transport or only Cl− self-exchange.
2. Physiological Role of NBCn1 in Breast Cancer
Experimental evidence supports the function of acid–base transports in cancer [11], while the function of NBCn1 in cancer and the pharmacological potential of inhibiting this transporter is still unclear. Recently, it was discovered that the presence of Nt truncated ErbB2 receptor (NErbB2) in the cancer cell line MCF-7 significantly upregulated NBCn1 expression. This overexpression increases cancer cells’ acid excretion ability, allowing them to lower the enormous intracellular acid burden induced by glycolytic metabolism and regulate pHi. This might work by the following mechanism: (1) full-length ErbB2 stimulates NBCn1 via the MEK/ERK1/2 and/or PI3K/AKT pathways; (2) The NErbB2 receptor activates Kruppel-like factor 4 (KLF4) through MEK/ERK1and/or PI3K/AKT1 pathways bind the NBCn1 promoter and increase mRNA and protein expression; and (3) NErbB2 promotes MEK/ERK2 and/or (Src) kinase to increase NBCn1 expression. Downregulation of another KLF4 superfamily member, Sp1, reduced NBCn1 via the aforementioned pathways, although the mechanism is unclear [12], as shown in Figure 2. It is indicated a substantial role for NBCn1 in ErbB2/HER2-positive breast cancers. With siRNA-knockdown investigations and pharmacologic inhibition, researchers found that NBCn1 is liable for the extrusion of acid in MCF-7 cells [11].
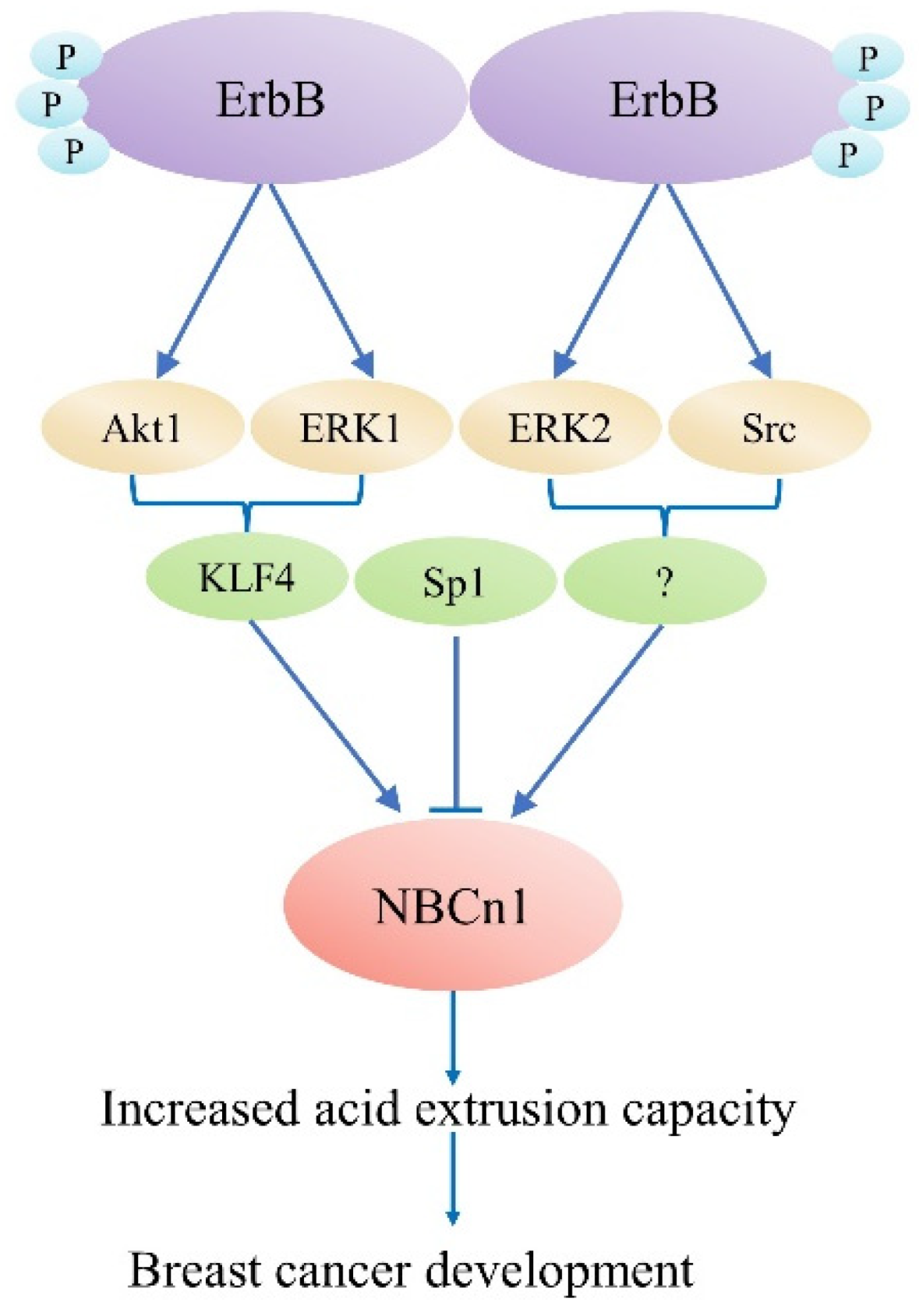
Figure 2. ErbB2-dependent SLC4A7 expression regulation model. The truncated ErbB2 receptor is responsible for regulating SLC4A7 expression.
Several recent genome-wide association studies have shown a correlation between breast cancer and genomic polymorphism in NBCn1, which raises the intriguing possibility that NBCn1 contributes to the etiology of human cancer [13][14][15]. Homozygosity for the single nucleotide polymorphism (SNP) rs4973768 was shown to increase the risk of breast cancer by 1.2–1.3 times [13][15]. A deeper study at the site of SNP suggests that it may modify the binding affinity for the microRNA miR-569, whose function in modulating NBCn1 expression levels is under investigation. NBCn1 contributes to breast cancer or acts as a modulator of cancer prognosis, as revealed by the finding that rs4973768 SNP is more common in breast cancer patients than in healthy controls. Recently, research identified that NBCn1 acts as a tyrosine kinase substrate during the progression of cancer [16], providing more evidence for the notion of NBCn1 in breast cancer. Remarkably, evidence from the MCF10AT family of breast cancer cell lines revealed that NBCn1 expression would decrease rather than increase as the disease progressed. Additional research, especially in tumor samples and in vivo, is required to define the significance of acid–base transporters.
3. Pharmacological Roles of NBCn1
Several different research organizations have been on the seek for pharmacological substances in order to conduct experiments that will identify the regulatory functions of NBCn1 cotransporters and for possible use in the treatment of disease. Some inhibitors of NBCn1 cotransport activity have been obtained, but these may be NBCs inhibitors rather than specific inhibitors of NBCn1. The lack of specificity prevents them from being widely used. In addition, these inhibitors may be used in experiments to monitor cellular function or to inhibit additional ion transport proteins (e.g., DIDS), which can make experiments difficult. However, these NBCs inhibitors can provide useful information on the molecular structure, which can interact with the NBCs cotransport proteins and may act as the initial point for the development of more specific therapeutic tools. The structure of these inhibitors is shown in Figure 3. Stilbene derivatives (e.g., DIDS, SITS) are extensively used NBCs inhibitors. However, the role of DIDS is more complex in the case of NBCn1. It was found that NBCn1 was unresponsive to DIDS even in the high micromolar range in mice mesenteric artery endothelial cells, as well as in choroid plexus and renal cells, while it was completely inhibited by DIDS in rat arterial smooth muscle cells. It might be due to the lack of an intact DIDS motif in NBCn1, resulting in lower DIDS sensitivity in certain cell types. To test this hypothesis, related RT-PCR products from NBCn1 expressed in mice mesenteric arteries were sequenced and reported to lack a complete DIDS motif [17]. Furthermore, the DIDS sensitivity of NBCn1 is enhanced when the KLFH sequence is mutated to KLFK [18].
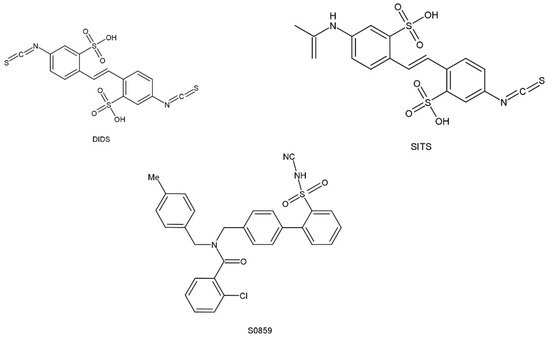
Figure 3. Chemical structural formula of NBCs inhibitor.
S0859, an N-cyanosulfonamide compound that acts as a generic NBCs inhibitor, is unable to distinguish between different Na+/HCO3− cotransport isoforms but shows significant improvement over stilbene derivatives. The preferred dose of S0859 in vitro was 15 μM, which provided about 90% inhibition of NBCs activity. Recently, S0859 exhibited inhibition of NBCn1 activity in MCF-7 human breast cancer cell lines [11]. S0859 is selective in acid–base transport in ventricular cardiomyocytes, where it does not impact Cl−/HCO3−, Cl−/OH−, or Na+/H+ exchange [19].
In addition, the application of inhibitory antibodies may be an effective way to inhibit NBCn1. Two investigations suggest antibodies may regulate Na+/HCO3− cotransport activity. In one research, NBCn1 activity in rat ventricular cardiomyocytes was successfully inhibited by a rabbit polyclonal antibody [20]. In another investigation, two antibodies against NBCe1 were prepared. One of these antibodies was able to suppress NBCe1 activity in cat ventricular cardiomyocytes, whereas the other antibody appeared to promote NBCe1 activity [21]. Antibodies that target specific proteins on cancer cells (such as anti-HER2 receptor antibodies) are effective in human clinical trials, providing a reason to believe that acid–base transporter inhibitory antibodies may find their way into clinical usage.
References
- Roos, A.; Boron, W.F. Intracellular pH. Physiol. Rev. 1981, 61, 296–434.
- Wakabayashi, S.; Shigekawa, M.; Pouyssegur, J. Molecular Physiology of Vertebrate Na+/H+ Exchangers. Physiol. Rev. 1997, 77, 51–54.
- Romero, M.F. Molecular pathophysiology of SLC4 bicarbonate transporters. Curr. Opin. Nephrol. Hypertens. 2005, 14, 495–501.
- Liu, Y.; Yang, J.; Chen, L.M. Structure and Function of SLC4 Family HCO3− Transporters. Front. Physiol. 2015, 6, 355.
- Wang, D.K.; Liu, Y.; Myers, E.J.; Guo, Y.M.; Xie, Z.D.; Jiang, D.Z.; Li, J.M.; Yang, J.; Liu, M.; Parker, M.D.; et al. Effects of Nt-truncation and coexpression of isolated Nt domains on the membrane trafficking of electroneutral Na+/HCO3− cotransporters. Sci. Rep. 2015, 5, 12241.
- Romero, M.F.; Fulton, C.M.; Boron, W.F. The SLC4 family of HCO3− transporters. Pflug. Arch.-Eur. J. Physiol. 2004, 447, 495–509.
- Thornell, I.M.; Bevensee, M.O. Regulators of Slc4 bicarbonate transporter activity. Front. Physiol. 2015, 6, 166.
- Aalkjaer, C.; Boedtkjer, E.; Choi, I.; Lee, S. Cation-coupled bicarbonate transporters. Compr. Physiol. 2014, 4, 1605–1637.
- Koltai, T.; Reshkin, S.J.; Harguindey, S. Carbonic anhydrases. In An Innovative Approach to Understanding and Treating Cancer: Targeting pH; Elsevier: Amsterdam, The Netherlands, 2020; pp. 157–176.
- Kurtz, I.; Zhu, Q. Structure, function, and regulation of the SLC4 NBCe1 transporter and its role in causing proximal renal tubular acidosis. Curr. Opin. Nephrol. Hypertens. 2013, 22, 572–583.
- Lauritzen, G.; Jensen, M.B.; Boedtkjer, E.; Dybboe, R.; Aalkjaer, C.; Nylandsted, J.; Pedersen, S.F. NBCn1 and NHE1 expression and activity in DeltaNErbB2 receptor-expressing MCF-7 breast cancer cells: Contributions to pHi regulation and chemotherapy resistance. Exp. Cell Res. 2010, 316, 2538–2553.
- Gorbatenko, A.; Olesen, C.W.; Morup, N.; Thiel, G.; Kallunki, T.; Valen, E.; Pedersen, S.F. ErbB2 upregulates the Na+,HCO3--cotransporter NBCn1/SLC4A7 in human breast cancer cells via Akt, ERK, Src, and Kruppel-like factor 4. FASEB J. 2014, 28, 350–363.
- Ahmed, S.; Thomas, G.; Ghoussaini, M.; Healey, C.S. Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat. Genet. 2009, 41, 585–590.
- Long, J.; Shu, X.O.; Cai, Q.; Gao, Y.T.; Zheng, Y.; Li, G.; Li, C.; Gu, K.; Wen, W.; Xiang, Y.B.; et al. Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2357–2365.
- Han, W.; Woo, J.H.; Yu, J.H.; Lee, M.J.; Moon, H.G.; Kang, D.; Noh, D.Y. Common genetic variants associated with breast cancer in Korean women and differential susceptibility according to intrinsic subtype. Cancer Epidemiol. Biomark. Prev. 2011, 20, 793–798.
- Chen, Y.; Choong, L.Y.; Lin, Q.; Philp, R.; Wong, C.H.; Ang, B.K.; Tan, Y.L.; Loh, M.C.; Hew, C.L.; Shah, N.; et al. Differential expression of novel tyrosine kinase substrates during breast cancer development. Mol. Cell Proteomics. 2007, 6, 2072–2087.
- Boedtkjer, E.; Praetorius, J.; Aalkjaer, C. NBCn1 (slc4a7) mediates the Na+-dependent bicarbonate transport important for regulation of intracellular pH in mouse vascular smooth muscle cells. Circ. Res. 2006, 98, 515–523.
- Choi, I.; Yang, H.; Kim, E.; Lee, S. Bicarbonate-Independent Sodium Conductance of Na/HCO3 Cotransporter NBCn1 Decreases NMDA Receptor Function. Curr. Issues Mol. Biol. 2022, 44, 1284–1293.
- Ch’en, F.F.; Villafuerte, F.C.; Swietach, P.; Cobden, P.M.; Vaughan-Jones, R.D. S0859, an N-cyanosulphonamide inhibitor of sodium-bicarbonate cotransport in the heart. Br. J. Pharmacol. 2008, 153, 972–982.
- Khandoudi, N.; Albadine, J.; Robert, P.; Krief, S.; Berrebi, I.; Martin, X.; Bevensee, M.O.; Boron, W.F.; Bril, A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc. Res. 2001, 52, 387–396.
- De Giusti, V.C.; Orlowski, A.; Villa-Abrille, M.C.; de Cingolani, G.E.; Casey, J.R.; Alvarez, B.V.; Aiello, E.A. Antibodies against the cardiac sodium/bicarbonate co-transporter (NBCe1) as pharmacological tools. Br. J. Pharmacol. 2011, 164, 1976–1989.
More
Information
Subjects:
Physiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
652
Revisions:
2 times
(View History)
Update Date:
13 Oct 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




