Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jianping Wu and Version 2 by Dean Liu.
Acid–base homeostasis is critical for proper physiological function and pathology. The SLC4 family of HCO3− transmembrane cotransporters is one of the HCO3− transmembrane transport carriers responsible for cellular pH regulation and the uptake or secretion of HCO3− in epithelial cells. NBCn1 (SLC4A7), an electroneutral Na+/HCO3− cotransporter, is extensively expressed in several tissues and functions as a cotransporter for net acid extrusion after cellular acidification.
- Na+/HCO3− cotransporter
- central nervous system
- pH
- cardiovascular system
1. Introduction
The maintenance of intracellular pH (pHi) and extracellular pH (pHo) is critical for biological function. The fluctuations in pH affect the enzymatic activity, as well as the function of the cell membrane and the signaling molecules, which inevitably contribute to modifications in cellular activity [1]. The Na+/H+ exchangers and the Na+-dependent/independent HCO3− transporters are the primary transporters that are responsible for mediating net acid extrusion in the majority of mammalian cells. HCO3− is one of the most essential acid–base buffer ions and is involved in regulating the pH of two gene families in vertebrates, invertebrates, and humans (SLC4 and SLC26) [2].
SLC4 consists of 10 genes, nine of which encode HCO3− cotransporters that are either Na+-independent (AEs) or Na+-dependent (NBCs) [3][4][3,4]. Until now, SLC4 family members are the only molecular entities functionally shown as Na+-dependent HCO3− transporters. The Na+-dependent HCO3− transporters are further divided into electrogenic cotransporters NBCe1 (SLC4A4) and NBCe2 (SLC4A5), electroneutral cotransporters NBCn1(SLC4A7) and NBCn2(SLC4A10), electrically neutral Na+-driven Cl−/HCO3− exchanger (NDCBE) [5][6][5,6]. Despite their obvious uniformity of more than 30% amino acid sequence identities between these proteins, NBCs vary in three major aspects, for example, ion selectivity, transportation pattern, and cellular localization.
The NBCs family is essential in the regulation of pH and the transdermal release and uptake of Na+, HCO3−, and Cl− in different tissues [7][8][7,8]. The mode of transport, along with membrane potential and ion gradient, is a key factor in determining the direction of transport. At resting membrane potential and normal ion gradient, HCO3− and Na+ are transported 1:1 or 2:1 into the cell and transported 3:1 out of the cell. In some pathophysiological conditions, such as ischemia, cancer, etc., the transmembrane ion gradient and membrane potential change significantly, which may affect the flow of transport as well as the activity of acid–base cotransporters. Within the cytosol and interstitial space near the transport site, the transmembrane transfer of HCO3− occurs in the chemical reaction CO2+H2O⇔HCO3−+H+, which is catalyzed by carbonic anhydrase [9]. When equilibrium is reached, the transport of HCO3− is equivalent to the transport of H+ in the opposite direction, as shown in Figure 1. NBCn1 transports Na+ and HCO3− with a stoichiometry of 1:1, whereas NBCe1 and NBCe2 can transport with a stoichiometry of 1:2 or 1:3, depending on the cell type and phosphorylation status [10]. NCDBE facilitates electroneutral Na+-dependent Cl−/HCO3− exchange. It remains controversial whether the Na+-dependent HCO3− transport mediated by NCBE/NBCn2 is coupled to net Cl− transport or whether it is solely related to Cl− self-exchange.
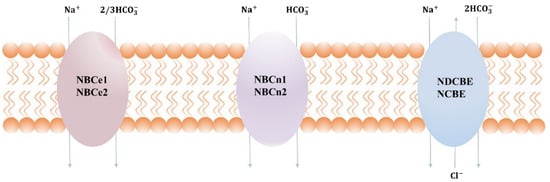
Figure 1. Transport stoichiometry of NBCs at intracellular (I) and extracellular (o) ion concentrations and membrane potentials. Note that NBCn2 and NCBE are the two alternative names suggested for SLC4A10, depending on whether the transporter mediates net Cl− transport or only Cl− self-exchange.
2. Physiological Role of NBCn1 in Breast Cancer
Experimental evidence supports the function of acid–base transports in cancer [11][73], while the function of NBCn1 in cancer and the pharmacological potential of inhibiting this transporter is still unclear. Recently, it was discovered that the presence of Nt truncated ErbB2 receptor (NErbB2) in the cancer cell line MCF-7 significantly upregulated NBCn1 expression. This overexpression increases cancer cells’ acid excretion ability, allowing them to lower the enormous intracellular acid burden induced by glycolytic metabolism and regulate pHi. This might work by the following mechanism: (1) full-length ErbB2 stimulates NBCn1 via the MEK/ERK1/2 and/or PI3K/AKT pathways; (2) The NErbB2 receptor activates Kruppel-like factor 4 (KLF4) through MEK/ERK1and/or PI3K/AKT1 pathways bind the NBCn1 promoter and increase mRNA and protein expression; and (3) NErbB2 promotes MEK/ERK2 and/or (Src) kinase to increase NBCn1 expression. Downregulation of another KLF4 superfamily member, Sp1, reduced NBCn1 via the aforementioned pathways, although the mechanism is unclear [12][74], as shown in Figure 24. It This study indicated a substantial role for NBCn1 in ErbB2/HER2-positive breast cancers. With siRNA-knockdown investigations and pharmacologic inhibition, researchers found that NBCn1 is liable for the extrusion of acid in MCF-7 cells [11][73].
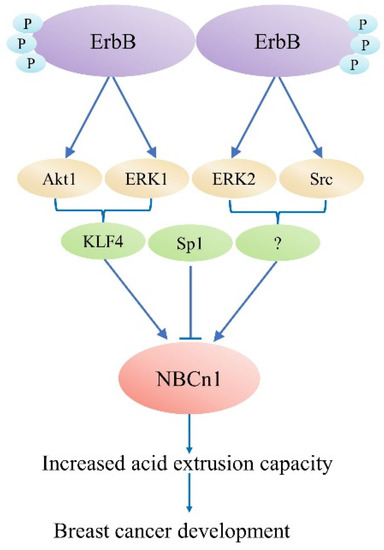
Figure 24. ErbB2-dependent SLC4A7 expression regulation model. The truncated ErbB2 receptor is responsible for regulating SLC4A7 expression.
Several recent genome-wide association studies have shown a correlation between breast cancer and genomic polymorphism in NBCn1, which raises the intriguing possibility that NBCn1 contributes to the etiology of human cancer [13][14][15][75,76,77]. Homozygosity for the single nucleotide polymorphism (SNP) rs4973768 was shown to increase the risk of breast cancer by 1.2–1.3 times [13][15][75,77]. A deeper study at the site of SNP suggests that it may modify the binding affinity for the microRNA miR-569, whose function in modulating NBCn1 expression levels is under investigation. NBCn1 contributes to breast cancer or acts as a modulator of cancer prognosis, as revealed by the finding that rs4973768 SNP is more common in breast cancer patients than in healthy controls. Recently, research identified that NBCn1 acts as a tyrosine kinase substrate during the progression of cancer [16][78], providing more evidence for the notion of NBCn1 in breast cancer. Remarkably, evidence from the MCF10AT family of breast cancer cell lines revealed that NBCn1 expression would decrease rather than increase as the disease progressed. Additional research, especially in tumor samples and in vivo, is required to define the significance of acid–base transporters.
3. Pharmacological Roles of NBCn1
Several different research organizations have been on the seek for pharmacological substances in order to conduct experiments that will identify the regulatory functions of NBCn1 cotransporters and for possible use in the treatment of disease. Some inhibitors of NBCn1 cotransport activity have been obtained, but these may be NBCs inhibitors rather than specific inhibitors of NBCn1. The lack of specificity prevents them from being widely used. In addition, these inhibitors may be used in experiments to monitor cellular function or to inhibit additional ion transport proteins (e.g., DIDS), which can make experiments difficult. However, these NBCs inhibitors can provide useful information on the molecular structure, which can interact with the NBCs cotransport proteins and may act as the initial point for the development of more specific therapeutic tools. The structure of these inhibitors is shown in Figure 35. Stilbene derivatives (e.g., DIDS, SITS) are extensively used NBCs inhibitors. However, the role of DIDS is more complex in the case of NBCn1. It was found that NBCn1 was unresponsive to DIDS even in the high micromolar range in mice mesenteric artery endothelial cells, as well as in choroid plexus and renal cells, while it was completely inhibited by DIDS in rat arterial smooth muscle cells. It might be due to the lack of an intact DIDS motif in NBCn1, resulting in lower DIDS sensitivity in certain cell types. To test this hypothesis, related RT-PCR products from NBCn1 expressed in mice mesenteric arteries were sequenced and reported to lack a complete DIDS motif [17][59]. Furthermore, the DIDS sensitivity of NBCn1 is enhanced when the KLFH sequence is mutated to KLFK [18][79].
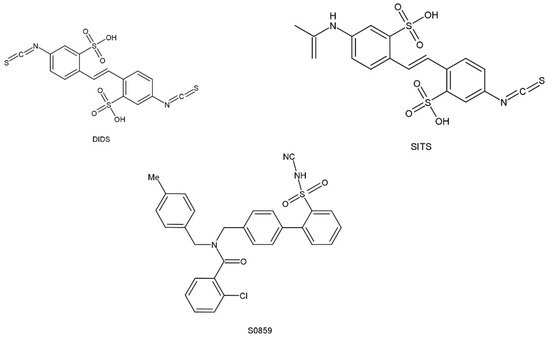
Figure 35. Chemical structural formula of NBCs inhibitor.
S0859, an N-cyanosulfonamide compound that acts as a generic NBCs inhibitor, is unable to distinguish between different Na+/HCO3− cotransport isoforms but shows significant improvement over stilbene derivatives. The preferred dose of S0859 in vitro was 15 μM, which provided about 90% inhibition of NBCs activity. Recently, S0859 exhibited inhibition of NBCn1 activity in MCF-7 human breast cancer cell lines [11][73]. S0859 is selective in acid–base transport in ventricular cardiomyocytes, where it does not impact Cl−/HCO3−, Cl−/OH−, or Na+/H+ exchange [19][80].
In addition, the application of inhibitory antibodies may be an effective way to inhibit NBCn1. Two investigations suggest antibodies may regulate Na+/HCO3− cotransport activity. In one research, NBCn1 activity in rat ventricular cardiomyocytes was successfully inhibited by a rabbit polyclonal antibody [20][81]. In another investigation, two antibodies against NBCe1 were prepared. One of these antibodies was able to suppress NBCe1 activity in cat ventricular cardiomyocytes, whereas the other antibody appeared to promote NBCe1 activity [21][82]. Antibodies that target specific proteins on cancer cells (such as anti-HER2 receptor antibodies) are effective in human clinical trials, providing a reason to believe that acid–base transporter inhibitory antibodies may find their way into clinical usage.
