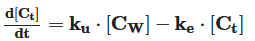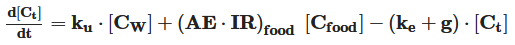| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Javier R. Viguri | -- | 1346 | 2022-09-05 14:53:04 | | | |
| 2 | Lindsay Dong | -28 word(s) | 1318 | 2022-09-06 03:37:59 | | | | |
| 3 | Lindsay Dong | + 3 word(s) | 1321 | 2022-09-06 03:39:36 | | | | |
| 4 | Lindsay Dong | -34 word(s) | 1287 | 2022-09-06 03:40:41 | | |
Video Upload Options
Sunscreens have become a product based on increasingly complex formulations that include, among many ingredients, a mixture of UV filters to provide optimal sun ultraviolet radiation protection. A significant group of scientific works deals with the impact of UV filters in aquatic media. However, the knowledge of the mechanism and kinetics of the compound’s direct release, fate, and its transformation and interaction with living organisms is necessary to assess its environmental occurrence and behavior and to predict potential and real impacts on the aquatic environment. The physical‐chemical properties, photodegradation, and release kinetics of particles and chemicals into the water are studied by hydrodynamic and kinetic models. Direct photolysis of chemicals is modeled as pseudofirst‐order kinetics, while the indirect pathway by the reaction of sunscreen with reactive oxygen species is described as second‐order kinetics. The interaction of UV filters with marine biota is studied mainly by toxicokinetic models, which predict their bio‐accumulation in the organisms’ tissues. These models consider the chemicals’ uptake and excretion, as well as their transfer between different internal animal organs, as a first‐order kinetic process.
1. Introduction
The analysis and modeling of the release, behavior, and fate of ingredients contained in sunscreen products when they come into contact with seawater allow scholars to predict their potential negative impacts on the marine environment. The development of a general modeling method, which allows scholars to model these complex (from an environmental point of view) matrices based on chemical cocktails, requires the minimization and management of the effects caused by the use of sunscreens in aquatic ecosystems.
2. Analysis and Modeling of the Behavior of Sunscreen’s Ingredients in an Aquatic Environment in the Absence of Organisms
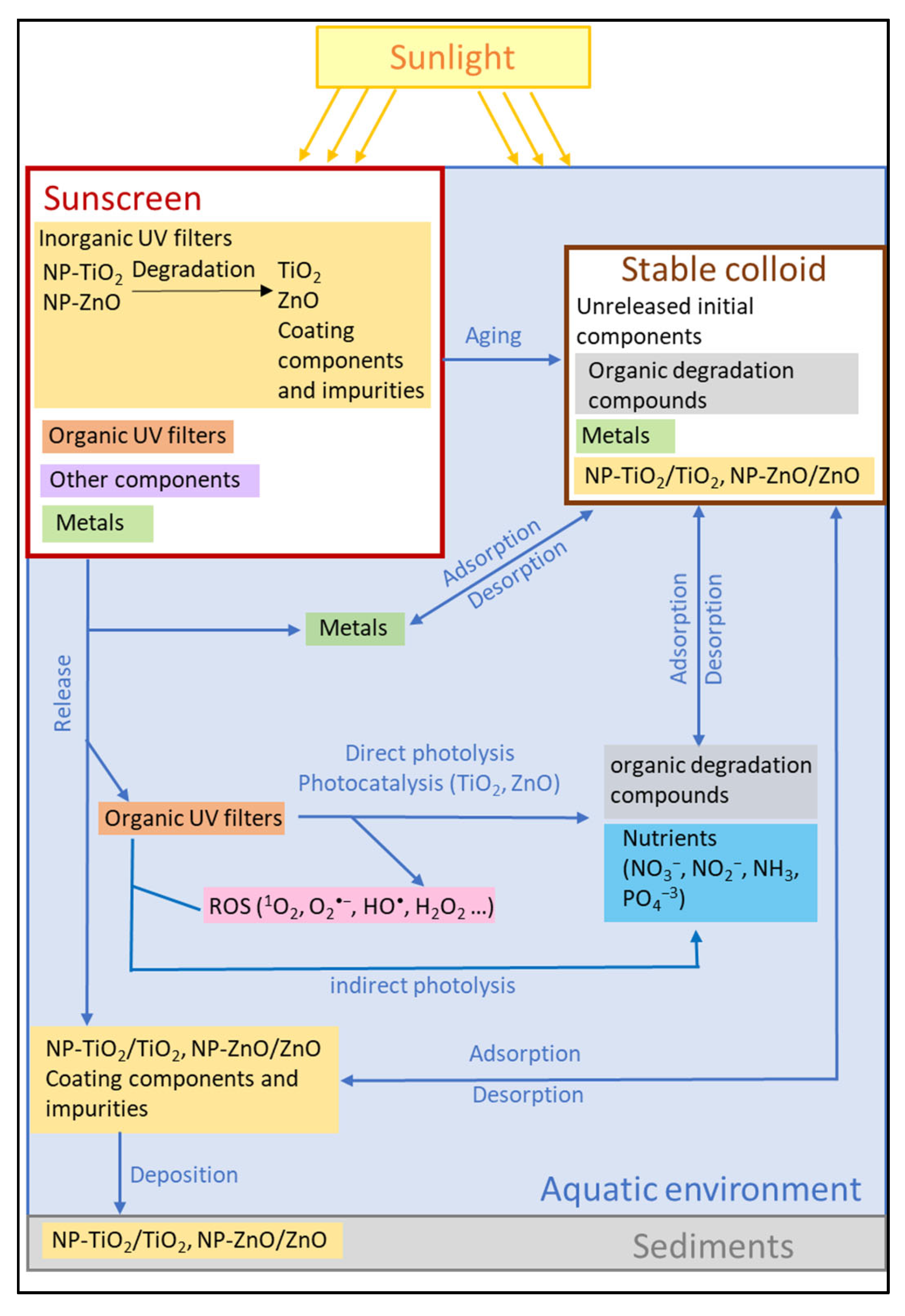
A large number of studies focus on the concentration and fate of sunscreen ingredients in different environment scenarios that are commonly used for recreational activities, such as rivers, lakes, estuaries, and beaches. Laboratory experiments aim to propose reaction mechanisms and kinetic models to describe the physico‐chemical transformations of sunscreen ingredients under controlled conditions. The results of such studies can be inputs to the model applied to real environmental scenarios. These works are analyzed in the following sections in the present work with Figure 1 showing the modeled transformations related to the release and transformation of the sunscreen ingredients in the aquatic environment in laboratory‐scale studies. Several works modeled and determined the photodegradation kinetics in laboratory‐simulated environmental conditions of organic UV filters. Several scholars studied the physicochemical processes related to TiO2‐NPs degradation and aging in aquatic media during a contact time of up to seven days using aqueous solutions of TiO2‐NPs. Organic UV‐filter degradation in aqueous media in the presence of TiO2‐NPs and light, as well as the degradation of nanoparticle surface coatings, can generate nutrients as nitrogen compounds (NO2−, NO3−, NH4+), phosphorous compounds (PO43−), and SiO2, which can have a considerable environmental impact.
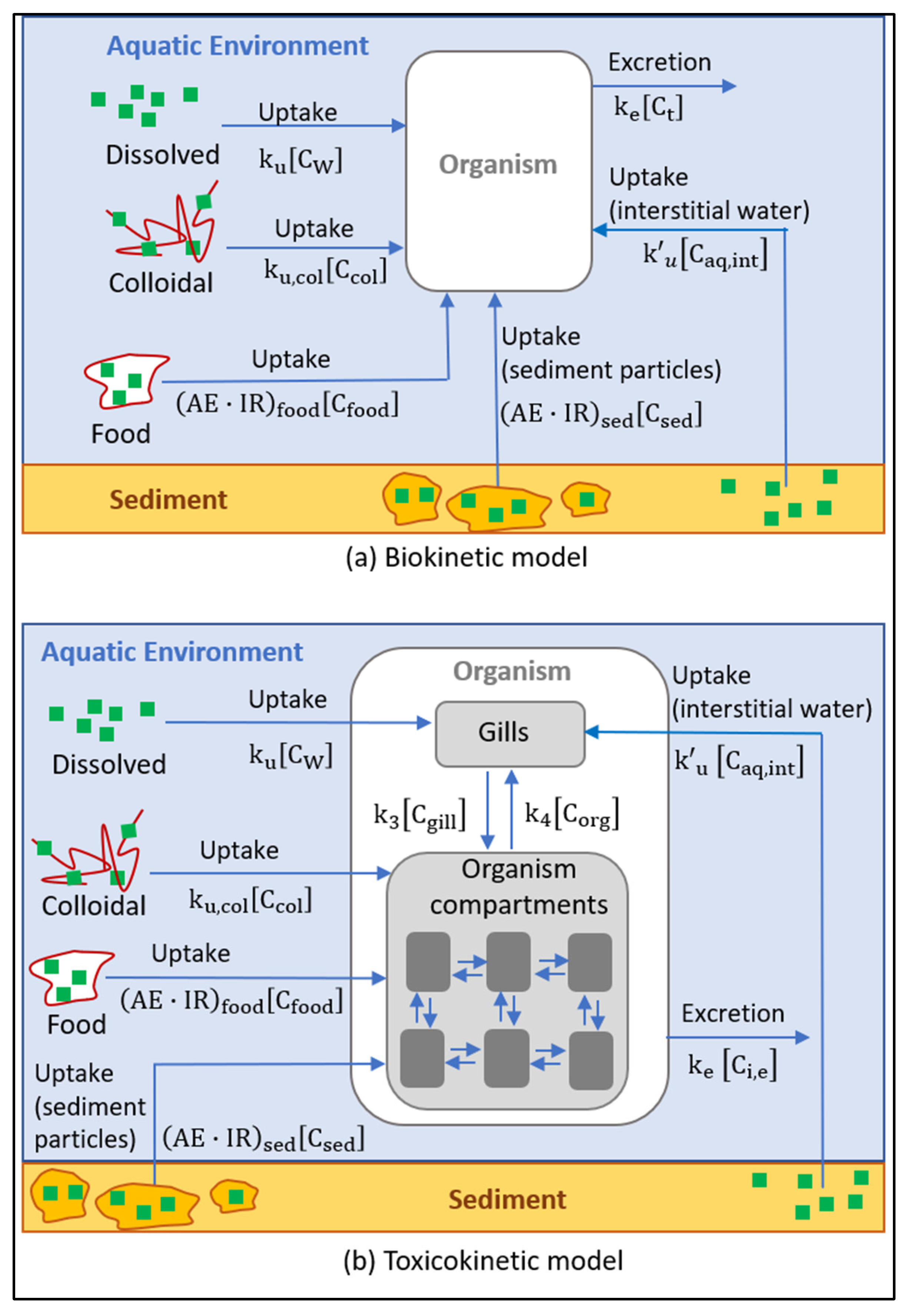
3. Analysis and Modeling of the Behavior of Sunscreen Ingredients in Contact with Aquatic Organisms
within the organism, the bioconcentration of the substance in the different compartments, and the interrelation between them.
|
|
UV Filters/Metals |
Water Type |
Field/Laboratory |
Reference |
|
|
|
|
|
|
|
Mussel |
4-MBC, BP3, BP4, OC, OD-PABA |
Seawater |
Laboratory |
[1] |
|
Fish |
Cu |
Fresh water |
Laboratory |
[2] |
|
Mussel |
EHMC |
Seawater |
Laboratory |
[3] |
|
Clams/mussels |
Zn |
Seawater |
Field |
[4] |
|
|
|
|
|
|
|
Oysters/mussels/clams |
Ag, Cd, Cr, Cu, Ni, Pb, Ti, Zn |
Seawater |
Field |
[5] |
|
Mussel |
As, Cd, Cr, Cu, Ni, Se, Pb, Zn |
Seawater |
Laboratory |
[6] |
|
Clams |
Ag, As, Zn |
Seawater |
Laboratory |
[7] |
|
Mussel |
Cd, Cr, Cu, Ni, Zn |
Fresh water |
Field |
[8] |
|
Scallop |
Cd, Zn |
Seawater |
Laboratory |
[9] |
|
Mussel |
Ag, Am, Cd, Co, Se, Zn |
Seawater |
Field |
[10] |
|
|
|
|
|
|
|
Clams/prawns/whiting |
Cd, Mn, Zn |
Seawater |
Laboratory |
[11] |
|
Oyster |
Zn |
Seawater |
Laboratory |
[12] |
4. Conclusions
Sunscreen has become a product widely used by people all over the world, both in everyday life and associated with sun and beach tourism. Since its invention, it has evolved through different stages to achieve optimal protection against the entire UV spectrum with increasingly complex formulations. However, restrictions of the use of certain components, due to the impact produced by their release and transformation in an aquatic environment, is a driver for the design of environmentally friendly formulations. The analysis and modeling of the release and behavior of sunscreen ingredients into the marine environment, including aquatic organisms, allowed scholars to predict the fate and levels of the chemicals under study, and therefore allowed scholars to predict the potential negative impacts on the aquatic environment. A large group of models studied the behavior of the particles and chemicals released into the water by hydrodynamic and kinetic models in the laboratory, while the knowledge of the fate of sunscreen components in the real environment is still limited. The laboratory‐scale works analyzed and model: (i) the physical–chemical property behavior of sunscreens once they are incorporated into the aquatic environment, (ii) the photodegradation of sunscreen components, and (iii) the release kinetics of metals present in sunscreens and the formation of nutrients in the receiving aquatic environment. The direct photolysis of sunscreens is modeled as pseudo‐first‐order kinetics, while the indirect pathway by the reaction of sunscreen with reactive oxygen species is described as second‐order kinetics. Another significant group of works analyzed the effect of the sunscreen components on marine biota (toxicokinetic models), which caused the bioaccumulation of certain compounds in the organisms’ tissues. These works considered that the processes of uptake and excretion of chemicals to and from organisms, as well as their transfer between different organs, followed a first‐order kinetic. The proposed models differ in the following ways: (i) the chemical uptake and (ii) the number of internal compartments of the organism considered, ranging from a single compartment to several compartments, such as the gills, digestive system, and internal organs. The kinetic parameters were obtained from the models for each species and the chemical allowed us to monitor the pollution levels in coastal areas, as well as the quality of the ecosystem.
The knowledge of the mechanisms of release and behaviour of the ingredients in sunscreens is essential to understand and predict their potential detrimental effects on marine ecosystems, as well as to identify their most environmentally harmful ingredients. The modeling approach was a useful prediction tool for these substances and can be of great value to environmental managers and stakeholders. However, the complexity (multitude of ingredients) and diversity (according to brand or format) of the sunscreen matrix, and the multitude of compartments and environmental variables that comprise the marine ecosystem, limited the application of these models. In this sense, future investigations should focus on the development of models, which consider the aforementioned factors, in order to obtain predictions of the potential impacts of these emerging products, and to inform future effective coastal‐protection strategies and conservation policies based on sustainable tourism.
References
- Vidal-Liñán, L.; Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Beiras, R. Bioaccumulation of UV filters in Mytilus galloprovincialis mussel. Chemosphere 2018, 190, 267–271.
- Chen, W.Y.; Liao, C.M. Interpreting copper bioaccumulation dynamics in tilapia using systems-level explorations of pulsed acute/chronic exposures. Ecotoxicology 2014, 23, 1124–1136.
- Gomez, E.; Bachelot, M.; Boillot, C.; Munaron, D.; Chiron, S.; Casellas, C.; Fenet, H. Bioconcentration of two pharmaceuticals (benzodiazepines) and two personal care products (UV filters) in marine mussels (Mytilus galloprovincialis) under controlled laboratory conditions. Environ. Sci. Pollut. Res. 2012, 19, 2561–2569.
- Chen, B.C.; Chen, W.Y.; Ju, Y.R.; Tsai, J.W.; Jou, L.J.; Singh, S.; Liao, C.M. Combining bioaccumulation and coping mechanism to enhance long-term site-specific risk assessment for zinc susceptibility of bivalves. Chemosphere 2011, 84, 707–715.
- Lu, G.; Zhu, A.; Fang, H.; Dong, Y.; Wang, W.X. Establishing baseline trace metals in marine bivalves in China and worldwide: Meta-analysis and modeling approach. Sci. Total Environ. 2019, 669, 746–753.
- Yin, Q.; Wang, W.X. Multiple trace element accumulation in the mussel Septifer virgatus: Counteracting effects of salinity on uptake and elimination. Environ. Pollut. 2018, 242, 375–382.
- Kalman, J.; Smith, B.D.; Bury, N.R.; Rainbow, P.S. Biodynamic modelling of the bioaccumulation of trace metals (Ag, As and Zn) by an in faunal estuarine invertebrate, the clam Scrobicularia plana. Aquat. Toxicol. 2014, 154, 121–130.
- Bourgeault, A.; Gourlay-Francéa, C.; Priadic, C.; Ayraultc, S.; Tusseau-Vuillemin, M.H. Bioavailability of particulate metal to zebra mussels: Biodynamic modelling shows that assimilation efficiencies are site-specific. Environ. Pollut. 2011, 159, 3381–3389.
- Pan, K.; Wang, W.X. Allometry of cadmium and zinc concentrations and bioaccumulation in the scallop Chlamys nobilis. Mar. Ecol. Prog. Ser. 2008, 365, 115–126.
- Wang, W.X.; Fisher, N.S.; Luoma, S.N. Kinetic determinations of trace element bioaccumulation in the mussel Mytilus edulis. Mar. Ecol. Prog. Ser. 1996, 140, 91–113.
- O’Mara, K.; Adams, M.; Burford, M.A.; Fry, B.; Cresswell, T. Uptake and accumulation of cadmium, manganese and zinc by fisheries species: Trophic differences in sensitivity to environmental metal accumulation. Sci. Total Environ. 2019, 690, 867–877.
- Lee, J.H.; Birch, G.F.; Cresswell, T.; Johansen, M.P.; Adams, M.S.; Simpson, S.L. Dietary ingestion of fine sediments and microalgae represent thedominant route of exposure and metal accumulation for Sydney rockoyster (Saccostrea glomerata): A biokinetic model for zinc. Aquat. Toxicol. 2015, 167, 46–54.