Sunscreens have become a product based on increasingly complex formulations that include,
among many ingredients, a mixture of UV filters to provide optimal sun ultraviolet radiation
protection. A significant group of scientific works deals with the impact of UV filters in aquatic
media. However, the knowledge of the mechanism and kinetics of the compound’s direct release,
fate, and its transformation and interaction with living organisms is necessary to assess its environmental
occurrence and behavior and to predict potential and real impacts on the aquatic environment.
This review outlines the existing analysis and modeling of the release and behavior of sunscreen’s
ingredients in the marine environment, including aquatic organisms. The physical‐chemical
properties, photodegradation, and release kinetics of particles and chemicals into the water are
studied by hydrodynamic and kinetic models. Direct photolysis of chemicals is modeled as pseudofirst‐
order kinetics, while the indirect pathway by the reaction of sunscreen with reactive oxygen
species is described as second‐order kinetics. The interaction of UV filters with marine biota is studied
mainly by toxicokinetic models, which predict their bio‐accumulation in the organisms’ tissues.
These models consider the chemicals’ uptake and excretion, as well as their transfer between different
internal animal organs, as a first‐order kinetic process. The studies analyzed in the present work
represent a driver of change for the beauty and personal care industry, in order to seek new ecological
alternatives through the application of R&D tactics.
- sunscreen
- chemicals
- modelling
- aquatic organism
- accumulation
1. Introduction
The analysis and modeling of the release, behavior, and fate of ingredients contained
in sunscreen products when they come into contact with seawater allow us to predict their
potential negative impacts on the marine environment. The development of a general
modeling method, which allows us to model these complex (from an environmental point
of view) matrices based on chemical cocktails, requires the minimization and management
of the effects caused by the use of sunscreens in aquatic ecosystems. In the present
work, we first present a brief perspective on sunscreen products. Secondly, we critically
review our current knowledge of models studied in the literature regarding the behavior
and transformation of these environmental harmful substances and their associated ingredients
in aquatic media. This review may represent an advance in the current state of
knowledge, given that it adds a different point of view on sunscreen release in aquatic
ecosystems, investigating the chemical behaviour of sunscreen compounds depending on
the experiments performed (in‐lab, in‐field, with organisms, without organisms). The
study also reports the mechanisms and kinetic models used, as well as the requirement
for the more precise and realistic modeling of skincare ingredients and, in general, for all
the emerging contaminants.
2. Analysis and Modeling of the Behavior of Sunscreen’s Ingredients in an Aquatic
Environment in the Absence of Organisms
The behavior, transformation, aging, and modeling of sunscreen’s ingredients in the
aquatic environment in the absence of organisms were identified through a systematic
literature review.
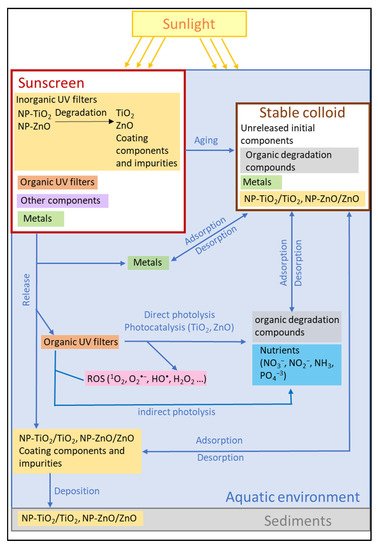
A large number of studies focus on the concentration and fate of sunscreen ingredients
in different environment scenarios that are commonly used for recreational activities,
such as rivers, lakes, estuaries, and beaches. Laboratory experiments aim to propose reaction mechanisms and kinetic models to
describe the physico‐chemical transformations of sunscreen ingredients under controlled
conditions. The results of such studies can be inputs to the model applied to real environmental
scenarios. These works are analyzed in the following sections in the present work with Figure
2 showing the modeled transformations related to the release and transformation of the
sunscreen ingredients in the aquatic environment in laboratory‐scale studies. Several works modeled and determined the photodegradation
kinetics in laboratory‐simulated environmental conditions of organic UV filters. Several authors studied the physicochemical processes related to TiO2‐NPs degradation
and aging in aquatic media during a contact time of up to seven days using aqueous
solutions of TiO2‐NPs. Organic UV‐filter degradation in aqueous media in the presence of TiO2‐NPs and
light, as well as the degradation of nanoparticle surface coatings, can generate nutrients
as nitrogen compounds (NO2−, NO3−, NH4+), phosphorous compounds (PO43−), and SiO2,
which can have a considerable environmental impact.
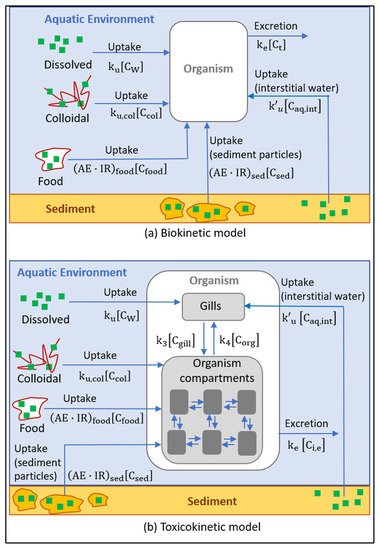
3. Analysis and Modeling of the Behavior of Sunscreen Ingredients in Contact with
Aquatic Organisms
release, transformation, and interaction with living aquatic organisms were identified
through a systematic literature review.
within the organism, the bioconcentration of the substance in the different compartments,
and the interrelation between them.

4. Conclusions
Sunscreen has become a product widely used by people all over the world, both in
everyday life and associated with sun and beach tourism. Since its invention, it has
evolved through different stages to achieve optimal protection against the entire UV spectrum
with increasingly complex formulations. However, restrictions of the use of certain
components, due to the impact produced by their release and transformation in an aquatic
environment, is a driver for the design of environmentally friendly formulations.
The analysis and modeling of the release and behavior of sunscreen ingredients into
the marine environment, including aquatic organisms, allowed us to predict the fate and
levels of the chemicals under study, and therefore allowed us to predict the potential negative
impacts on the aquatic environment. A large group of models studied the behavior
of the particles and chemicals released into the water by hydrodynamic and kinetic models
in the laboratory, while the knowledge of the fate of sunscreen components in the real
environment is still limited. The laboratory‐scale works analyzed and model: (i) the physical–
chemical property behavior of sunscreens once they are incorporated into the aquatic
environment, (ii) the photodegradation of sunscreen components, and (iii) the release kinetics
of metals present in sunscreens and the formation of nutrients in the receiving
aquatic environment. The direct photolysis of sunscreens is modeled as pseudo‐first‐order
kinetics, while the indirect pathway by the reaction of sunscreen with reactive oxygen
species is described as second‐order kinetics.
Another significant group of works analyzed the effect of the sunscreen components
on marine biota (toxicokinetic models), which caused the bioaccumulation of certain compounds
in the organisms’ tissues. These works considered that the processes of uptake
and excretion of chemicals to and from organisms, as well as their transfer between different
organs, followed a first‐order kinetic. The proposed models differ in the following
ways: (i) the chemical uptake and (ii) the number of internal compartments of the organism
considered, ranging from a single compartment to several compartments, such as the
gills, digestive system, and internal organs. The kinetic parameters were obtained from
the models for each species and the chemical allowed us to monitor the pollution levels in
coastal areas, as well as the quality of the ecosystem.
The knowledge of the mechanisms of release and behaviour of the ingredients in
sunscreens is essential to understand and predict their potential detrimental effects on
marine ecosystems, as well as to identify their most environmentally harmful ingredients.
The modeling approach was a useful prediction tool for these substances and can be of
great value to environmental managers and stakeholders. However, the complexity (multitude
of ingredients) and diversity (according to brand or format) of the sunscreen matrix,
and the multitude of compartments and environmental variables that comprise the marine
ecosystem, limited the application of these models. In this sense, future investigations
should focus on the development of models, which consider the aforementioned factors,
in order to obtain predictions of the potential impacts of these emerging products, and to
inform future effective coastal‐protection strategies and conservation policies based on
sustainable tourism.
This entry is adapted from the peer-reviewed paper 10.3390/oceans3030024
