Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Peipei Wang | -- | 167 | 2022-08-22 07:07:41 | | | |
| 2 | Peipei Wang | + 1373 word(s) | 1540 | 2022-08-22 07:16:32 | | | | |
| 3 | Jessie Wu | + 8 word(s) | 1548 | 2022-08-23 07:41:45 | | | | |
| 4 | Jessie Wu | + 1 word(s) | 1549 | 2022-08-23 07:43:25 | | | | |
| 5 | Jessie Wu | Meta information modification | 1549 | 2022-08-23 07:46:19 | | | | |
| 6 | Jessie Wu | Meta information modification | 1549 | 2022-08-23 07:46:57 | | | | |
| 7 | Jessie Wu | Meta information modification | 1549 | 2022-08-23 07:49:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Qi, M.; Zheng, C.; Wu, W.; Yu, G.; Wang, P. The Biological Activities of Marine Exopolysaccharides. Encyclopedia. Available online: https://encyclopedia.pub/entry/26345 (accessed on 13 January 2026).
Qi M, Zheng C, Wu W, Yu G, Wang P. The Biological Activities of Marine Exopolysaccharides. Encyclopedia. Available at: https://encyclopedia.pub/entry/26345. Accessed January 13, 2026.
Qi, Mingxing, Caijuan Zheng, Wenhui Wu, Guangli Yu, Peipei Wang. "The Biological Activities of Marine Exopolysaccharides" Encyclopedia, https://encyclopedia.pub/entry/26345 (accessed January 13, 2026).
Qi, M., Zheng, C., Wu, W., Yu, G., & Wang, P. (2022, August 22). The Biological Activities of Marine Exopolysaccharides. In Encyclopedia. https://encyclopedia.pub/entry/26345
Qi, Mingxing, et al. "The Biological Activities of Marine Exopolysaccharides." Encyclopedia. Web. 22 August, 2022.
Copy Citation
The unique living environment of marine microorganisms endows them with the potential to produce novel compounds with diverse biological activities. Exopolysaccharide (EPS) is a high molecular weight carbohydrate polymer secreted by microorganisms during growth and metabolism. The complex and diverse structures of EPS endow them with unique biological activities and functions.
exopolysaccharides
marine
antioxidant activity
1. Introduction
The structure of the marine microbial Exopolysaccharides (EPS) is complex and diverse [1], and is linked to various biological activities, such as antibacterial, antioxidant, anti-cancer, antifreeze, anti-inflammatory, enhancement of immune activity and blood pressure, and lipid reduction [2][3][4][5]. In addition, due to the particularity of the marine microbial environment, the EPS produced by marine microbials also have a potential application value in the marine ecological environment. Here, researchers mainly introduced the antioxidant activity, anticancer activity, anti-infectious activity, and immune-enhancing biological activity of the marine microbial EPS (as shown in Figure 1) and their potential application in bioremediation and carbon sequestration.
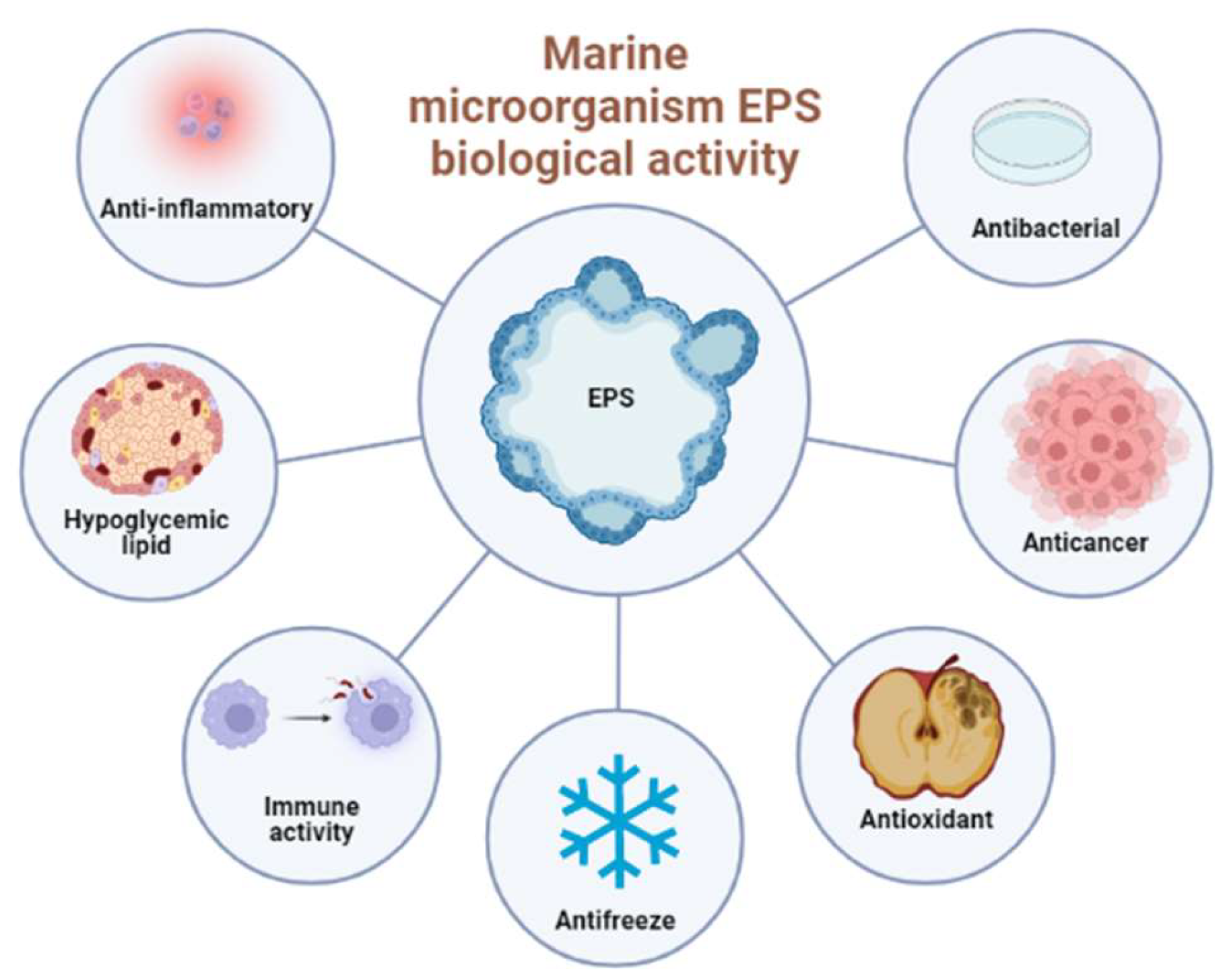
Figure 1. Main biological activities of marine microbial EPS.
2. Antioxidant Activity
Oxygen is the key substance in the normal life-metabolism of aerobic organisms [6]. In the metabolism of organisms, the living organisms inevitably produce reactive oxygen species (ROS) [7]. High levels of ROS may disrupt the pro-oxidant/antioxidant balance in organisms, leading to oxidative stress [8]; excessive ROS will destroy the normal function of lipids, proteins, and DNA in human cells, thus inducing various diseases [9]. Several pieces of evidence have proved that antioxidants play an important role in protecting humans from cancer, diabetes, cardiovascular disease, and neurodegenerative diseases related to different types of oxidative damage [10][11][12].
EPS from marine bacteria generally has strong antioxidant activity, which is related to the structural features of EPS, including sulfate content and its binding sites, monosaccharide residues, and glycosidic bonds [13]. Marine bacterial EPS often contributes to the formation of biofilms, which adapt to extreme environments, such as high salinity, low temperature, and high osmotic pressure [14][15][16]. For example, AEPS of Rhodella reticulata has a stronger scavenging ability for superoxide anion than the standard antioxidant α-tocopherol [17]. EPS sp isolated from Polaris marine arcticum. SM1127 has good antioxidant capacity. The antioxidant capacity is significantly higher than that of hyaluronic acid (HA), a common free radical scavenging binder in cosmetics, which indicates that this EPS has a good application prospect in the field of cosmetic antioxidants in the future [18]. In addition, SM1127 can remove excess reactive oxygen species produced by wound infection and inflammation, thereby accelerating wound healing. Therefore, this EPS is likely to be used to accelerate the healing of frostbite, burns and other wounds [19]. Wu et al. reported that the marine bacterium EPS EPS27 produced by P. stutzeri 273 had a good hydroxyl radical scavenging rate, up to 70% when the EPS concentration was 60 μg/mL. Therefore, EPS27 has good antioxidant activity and has potential applications in food and health care fields [20]. The skin is the largest organ of the human body, and skin wound healing is an important clinical problem [21][22][23]. Since synthetic drugs carry a high risk of side effects, such as allergy and drug resistance, natural products such as EPS are becoming increasingly important and are strongly recommended as alternative medicines for wound healing.
Another source of antioxidants is EPS produced by marine fungi. Wang et al. A novel extracellular polysaccharide (YSS), consisting of Man and Gal units with a molecular weight of 18.6 kDa, was discovered from the marine fungus Aspergillus kawachi [24]. YSS has a strong scavenging ability to DPPH free radical with EC50 of 2.8 mg/mL. Chen et al. It has been reported that the marine fungus Fusarium oxysporum produces a novel galactofuranos-containing EPS Fw-1, mainly composed of Gal, Glc and Man, with a molecular weight of 61.2 kDa [25]. The EC50 of Fw-1 for hydroxyl and superoxide radical scavenging was 1.1 and 2.0 mg/mL, respectively, which was greater than that of the EPS A. versicolor LCJ-5-4 named AVP isolated from the ocean ( EC50 of 4.0 mg/mL ). The antioxidant EPS monosaccharide extracted from marine fungi is relatively simple in composition and small in molecular weight, which is more suitable for studying the relationship between marine polysaccharide structure and antioxidants [26][27].
Epidemiological investigations have demonstrated a strong correlation between antioxidant utilization and reduced risk of common chronic diseases such as cardiovascular disease and cancer. Compared with many reports on the antioxidant activity of marine microbial EPS, there are few reports on the isolation, purification and structural analysis of marine microbial EPS. It is necessary to study their structure-activity relationship in depth.
3. Anticancer Activity
Now, new sources of nontoxic natural substances with potential anticancer effects are being actively researched [28]. In the past decade, there has been great interest in developing anticancer polysaccharide drugs. Marine microorganisms have unique metabolic and physiological capabilities that enable them to produce various biological compounds [29][30][31], such as EPS. Several marine microbial EPS have been reported to have anticancer activity through mitochondrial dysfunction, inhibition of cell proliferation, or modulation of the immune system [32][33][34][35][36][37][38]. Matsuda et al. investigated marine Pseudomonas polysaccharide B1 and found that it could induce apoptosis in U937 cells [38]. Chen et al. The Antarctic bacterium Pseudoaltermonas sp has been reported . S-5 produces a hetero-exopolysaccharide (called PEP) that significantly inhibits the growth of human leukemia cell K562 [33]. Additionally, Ramamoorthy Sathishkumar et al. Discovery of EPS from ascidian symbiotic Bacillus thuringiensis It has good anticancer activity in vitro. Compared to normal Vero cells, this polysaccharide showed potential cytotoxicity against cancer cell lines A549 and HEP-2. The inhibitory rate of EPS on both cancer cell lines increased in a dose-dependent manner [32]. AS2-1 produced by Alternaria . The growth of Hela, HL-60 and K562 cells can also be inhibited in a concentration-dependent manner [39]. The marine bacterial exopolysaccharide EPS11 can effectively inhibit the adhesion, migration and invasion of hepatoma cells; this potential target protein and molecular mechanism were first explored by targeting the β1-integrin signaling pathway of type I collagen [36]. Recently, a study showed that newly isolated marine bacterial EPS could enhance the antitumor activity of HepG2 cells by affecting key apoptotic factors and activating toll-like receptors (TLRs) [40]. Other studies have shown that chemical modifications of EPS, such as acetylation, carboxymethylation, and sulfonation, can also enhance its biological activity [41][42], which in turn enhances its anticancer activity. Maza et al. Two polysaccharides, EPS-DR and EPS-DRS, were demonstrated to form complexes with scandium, and these complexes displayed various biological activities, especially antiproliferative properties in cancer cells.
4. Anti-infectious Disease
EPS also plays an important role in fighting infectious diseases. Numerous studies have shown that the immunological and antiviral activities of marine microbial EPS have potential value in inhibiting certain influenza viruses and bacteria [43]. EPS, as a potent antibacterial agent, mainly inhibits bacterial growth by inhibiting biofilm formation. Mihlid H et al. Enterobacteriaceae are reported. ACD2 EPS from Tabuk region of Saudi Arabia showed some inhibitory effect on Escherichia coli and Staphylococcus aureus [44]. Durairajan Rubini et al. reported a marine polysaccharide with good antibacterial activity and strong inhibitory effect against uropathogenic Escherichia coli (UPEC), providing an antibiotic-free approach for the treatment of urinary tract infections [45]. Similarly, Wu et al. The exopolysaccharide EPS273 from the culture supernatant of the marine bacterium P. stutzeri 273 was reported to inhibit P. aeruginosa via anti-biofilm activity [20]. It is not only effective against animal bacteria but also plant bacteria. Marwa Drira et al. EPS produced by Porphyridium sordidum was found to lead to plant control of fungal growth, and EPS could act as an inducer to enhance the resistance of Arabidopsis to Fusarium oxysporum [46]. In addition, sulfated EPS from Porphyridium sp. Antiviral effects have been shown against herpesviruses (HSV-1 and HSV-2) [47][48].
5. Immunomodulatory Activity
The main function of the immune system is to recognize and eliminate pathogens to maintain physiological balance and stability [49]. When immunity is compromised, various adverse immune responses result [50]. Several EPSs synthesized by marine microorganisms have immunomodulatory activities [19][51][52][53][54][55][56]. For example, an EPS called EPS2E1 was extracted from the marine Halomonas sp. And showed good immune-enhancing activity, mainly by activating MAPK and NF-κB pathways [57]. Soumya Chatterjee et al. reported Sphingobacter sp., a new α-mannan EPS from Sphingobacter arcticum. IITKGP-BTPF3 significantly reduced LPS-induced NO production in macrophages. These results suggest that sphingosine has a potential activating effect on the anti-inflammatory effects of macrophages in vitro [58]. On the one hand, there is evidence that marine microbial EPS can induce the expression of cytokines, such as interleukin (IL), tumor necrosis factor (IF-α), and interferon [59]. Additionally, Adriana et al. It has been reported that EPS-1 may help improve immune surveillance of PBMCs against viral infection by inducing polarization in favor of Th1 subsets. Bacillus licheniformisProduced EPS-1 induces cytokine production to enhance immune regulation [54]. It mainly promotes macrophage secretion of mediators and enzymes, such as NO, COX-2, IL-1, IL-6 and TNF-α, which play an important role in mediating inflammation and tissue repair in RAW264.7 macrophages [60][61][62][63]. YCP, a native EPS derived from the mycelium of the marine filamentous fungus Phoma herbarum YS4108 , binds to TLR-2 and TLR-4, and is expressed by T cells and dendritic cells (DC) It has great antitumor potential due to specific immunomodulatory ability mediated [64]. Recently, a novel EPS (AUM-1) SCAU-266 was obtained from marine Aureobasidium melanogenum with potential effects on ferroptosis-related immunomodulatory properties in RAW264.7 cells. Mechanistic studies have shown that due to higher levels of reactive oxygen species in glutamate metabolism and TCA cycle, it can regulate the expression of GPX4, regulate glutathione (oxidation), and directly cause lipid peroxidation ( Figure 2 ) [53].
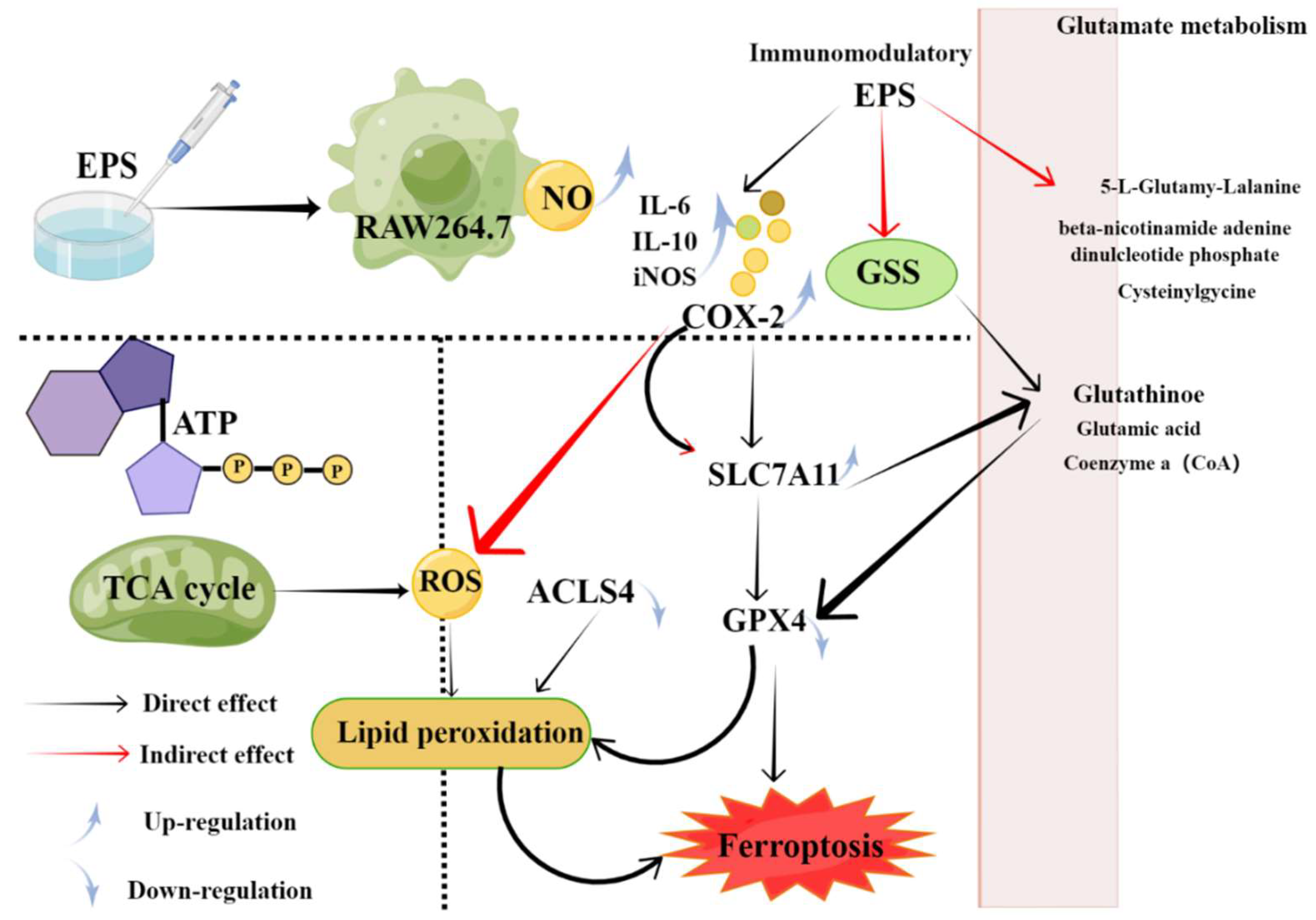
Figure 2. Potential mechanisms of ferroptosis-related immune regulation by AUM-1 [53].
References
- Wang, Y.Z.; Li, H.; Dong, F.K.; Yan, F.; Cheng, M.; Li, W.Z.; Chang, Q.; Song, T.Z.; Liu, A.Y.; Song, B. Therapeutic Effect of Calcipotriol Pickering Nanoemulsions Prepared by Exopolysaccharides Produced by Bacillus halotolerans FYS Strain on Psoriasis. Int. J. Nanomed. 2020, 15, 10371–10384.
- Besednova, N.N.; Smolina, T.P.; Andryukov, B.G.; Kuznetsova, T.A.; Mikhailov, V.V.; Zvyagintseva, T.N. Exopolysaccharides of Marine Bacteria: Prospects for Use in Medicine. Antibiot. Khimiote. 2018, 63, 67–78.
- Hassan, S.W.M.; Ibrahim, H.A.H. Production, Characterization and Valuable Applications of Exopolysaccharides from Marine Bacillus subtilis SH1. Pol. J. Microbiol. 2017, 66, 449–461.
- Wu, M.H.; Pan, T.M.; Wu, Y.J.; Chang, S.J.; Chang, M.S.; Hu, C.Y. Exopolysaccharide activities from probi-otic bifidobacterium: Immunomodulatory effects (on J774A.1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 2010, 144, 104–110.
- Yeh, M.-Y.; Ko, W.-C.; Lin, L.-Y. Hypolipidemic and Antioxidant Activity of Enoki Mushrooms (Flammulina velutipes). BioMed. Res. Int. 2014, 2014, 1–6.
- Li, H.; Ding, F.; Xiao, L.; Shi, R.; Wang, H.; Han, W.; Huang, Z. Food-Derived Antioxidant Polysaccharides and Their Pharmacological Potential in Neurodegenerative Diseases. Nutrients 2017, 9, 778.
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H.; Li, H. Overview of Antioxidant Peptides Derived from Marine Resources: The Sources, Characteristic, Purification, and Evaluation Methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxida-tive stress-induced cancer. Chem. Biol. Interact 2006, 160, 1–40.
- Acharya, A.; Das, I.; Chandhok, D.; Saha, T. Redox regulation in cancer A double-edged sword with therapeu-tic potential. Oxid. Med. Cell Longev. 2010, 3, 23–34.
- Lin, M.T.; Beal, M.F. The oxidative damage theory of aging. Clin. Neurosci. Res. 2003, 2, 305–315.
- Pereira, M.D.; Ksiazek, K.; Menezes, R. Oxidative Stress in Neurodegenerative Diseases and Ageing. Oxidative Med. Cell. Longev. 2012, 2012, 376–385.
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74.
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487.
- Squillaci, G.; Finamore, R.; Diana, P.; Restaino, O.F.; Schiraldi, C.; Arbucci, S.; Ionata, E.; La Cara, F.; Morana, A. Production and properties of an exopolysaccharide synthesized by the extreme halophilic archaeon Haloterrigena turkmenica. Appl. Microbiol. Biot. 2016, 100, 613–623.
- Xiao, R.; Yang, X.; Li, M.; Li, X.; Wei, Y.; Cao, M.; Ragauskas, A.; Thies, M.; Ding, J.; Zheng, Y. Investigation of composition, structure and bioactivity of extracellular polymeric substances from original and stress-induced strains of Thraustochytrium striatum. Carbohydr. Polym. 2018, 195, 515–524.
- Sun, M.-L.; Liu, S.-B.; Qiao, L.-P.; Chen, X.-L.; Pang, X.; Shi, M.; Zhang, X.-Y.; Qin, Q.-L.; Zhou, B.-C.; Zhang, Y.-Z.; et al. A novel exopolysaccharide from deep-sea bacterium Zunongwangia profunda SM-A87: Low-cost fermentation, moisture retention, and antioxidant activities. Appl. Microbiol. Biotechnol. 2014, 98, 7437–7445.
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2009, 26, 833–840.
- Liu, S.-B.; Chen, X.-L.; He, H.-L.; Zhang, X.-Y.; Xie, B.-B.; Yu, Y.; Chen, B.; Zhou, B.-C.; Zhang, Y.Z. Structure and Ecological Roles of a Novel Exopolysaccharide from the Arctic Sea Ice Bacterium Pseudoalteromonas sp. Strain SM20310. Appl. Environ. Microbiol. 2013, 79, 224–230.
- Wang, Q.; Wei, M.; Zhang, J.; Yue, Y.; Wu, N.; Geng, L.; Sun, C.; Zhang, Q.; Wang, J. Structural characteristics and immune-enhancing activity of an extracellular polysaccharide produced by marine Halomonas sp. 2E1. Int. J. Biol. Macromol. 2021, 183, 1660–1668.
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and Anti-Infection of a Marine Bacterial Exopolysaccharide Against Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 102.
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366.
- Gautam, S.; Chou, C.F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Surface modification of nanofibrous poly-caprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Mat. Sci. Eng. C Mater. 2014, 34, 402–409.
- Zhou, X.; Ning, K.; Ling, B.; Chen, X.; Cheng, H.; Lu, B.; Gao, Z.; Xu, J. Multiple Injections of Autologous Adipose-Derived Stem Cells Accelerate the Burn Wound Healing Process and Promote Blood Vessel Regeneration in a Rat Model. Stem Cells Dev. 2019, 28, 1463–1472.
- Chen, Y.; Mao, W.; Gao, Y.; Teng, X.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; et al. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr. Polym. 2013, 93, 478–483.
- Chen, Y.-L.; Mao, W.-J.; Tao, H.-W.; Zhu, W.-M.; Yan, M.-X.; Liu, X.; Guo, T.-T.; Guo, T. Preparation and Characterization of a Novel Extracellular Polysaccharide with Antioxidant Activity, from the Mangrove-Associated Fungus Fusarium oxysporum. Mar. Biotechnol. 2015, 17, 219–228.
- Hamidi, M.; Kozani, P.S.; Kozani, P.S.; Pierre, G.; Michaud, P.; Delattre, C. Marine Bacteria versus Microalgae: Who Is the Best for Biotechnological Production of Bioactive Compounds with Antioxidant Properties and Other Biological Applications? Mar. Drugs. 2020, 18, 28.
- Petruk, G.; Roxo, M.; De Lise, F.; Mensitieri, F.; Notomista, E.; Wink, M.; Izzo, V.; Monti, D.M. The marine Gram-negative bacterium Novosphingobium sp. PP1Y as a potential source of novel metabolites with antioxidant activity. Biotechnol. Lett. 2018, 41, 273–281.
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Mar. Drugs. 2013, 11, 4876–4901.
- Bhatnagar, I.; Kim, S.-K. Immense Essence of Excellence: Marine Microbial Bioactive Compounds. Mar. Drugs 2010, 8, 2673–2701.
- De Carvalho, C.C.C.R.; Fernandes, P. Production of Metabolites as Bacterial Responses to the Marine Environment. Mar. Drugs. 2010, 8, 705–727.
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol. Adv. 2010, 28, 436–450.
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anti-cancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120.
- Caruso, C.; Rizzo, C.; Mangano, S.; Poli, A.; Di Donato, P.; Nicolaus, B.; Finore, I.; Di Marco, G.; Michaud, L.; Giudice, A.L. Isolation, characterization and optimization of EPSs produced by a cold-adapted Marinobacter isolate from Antarctic seawater. Antarct. Sci. 2019, 31, 69–79.
- Parra-Riofrío, G.; García-Márquez, J.; Casas-Arrojo, V.; Uribe-Tapia, E.; Abdala-Díaz, R. Antioxidant and Cytotoxic Effects on Tumor Cells of Exopolysaccharides from Tetraselmis suecica (Kylin) Butcher Grown Under Autotrophic and Heterotrophic Conditions. Mar. Drugs 2020, 18, 534.
- Aullybux, A.A.; Puchooa, D.; Bahorun, T.; Jeewon, R.; Wen, X.S.; Matin, P. Antioxidant and Cytotoxic Activities of Exopolysaccharides from Alcaligenes faecalis Species Isolated from the Marine Environment of Mauritius. J. Polym. Environ. 2022, 30, 1462–1477.
- Liu, G.; Liu, R.; Shan, Y.; Sun, C. Marine bacterial exopolysaccharide EPS11 inhibits migration and invasion of liver cancer cells by directly targeting collagen I. J. Biol. Chem. 2021, 297.
- Cao, C.; Li, Y.; Wang, C.; Zhang, N.; Zhu, X.; Wu, R.; Wu, J. Purification, characterization and antitumor activity of an exopolysaccharide produced by Bacillus velezensis SN-1. Int. J. Biol. Macromol. 2020, 156, 354–361.
- Matsuda, M.; Yamori, T.; Naitoh, M.; Okutani, K. Structural Revision of Sulfated Polysaccharide B-1 Isolated from a Marine Pseudomonas Species and Its Cytotoxic Activity Against Human Cancer Cell Lines. Mar. Biotechnol. 2003, 5, 13–19.
- Chen, Y.; Mao, W.-J.; Yan, M.-X.; Liu, X.; Wang, S.-Y.; Xia, Z.; Xiao, B.; Cao, S.-J.; Yang, B.-Q.; Li, J. Purification, Chemical Characterization, and Bioactivity of an Extracellular Polysaccharide Produced by the Marine Sponge Endogenous Fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313.
- Yahya, S.M.M.; Abdelnasser, S.M.; Hamed, A.R.; El Sayed, O.H.; Asker, M.S. Newly isolated marine bacterial exopolysaccharides enhance antitumor activity in HepG2 cells via affecting key apoptotic factors and activating toll like receptors. Mol. Biol. Rep. 2019, 46, 6231–6241.
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179.
- Chaisuwan, W.; Jantanasakulwong, K.; Wangtueai, S.; Phimolsiripol, Y.; Chaiyaso, T.; Techapun, C.; Phongthai, S.; You, S.; Regenstein, J.M.; Seesuriyachan, P. Microbial exopolysaccharides for immune enhancement: Fermentation, modifications and bioactivities. Food Bio. Sci. 2020, 35, 100564.
- Tang, Y.; Cui, Y.; De Agostini, A.; Zhang, L. Chapter Eighteen—Biological mechanisms of glycan and glycosaminoglycan based nutraceuticals. Prog. Mol. Biol. Transl. Sci. 2019, 163, 445–469.
- Almutairi, M.H.; Helal, M.M. Biological and microbiological activities of isolated Enterobacter sp. ACD2 exopolysaccharides from Tabuk region of Saudi Arabia. J. King Saud Univ. Sci. 2020, 33, 101328.
- Rubini, D.; Varthan, P.V.; Jayasankari, S.; Vedahari, B.N.; Nithyanand, P. Suppressing the phenotypic virulence factors of Uropathogenic Escherichia coli using marine polysaccharide. Microb Pathog. 2020, 141, 103973.
- Drira, M.; Elleuch, J.; Ben Hlima, H.; Hentati, F.; Gardarin, C.; Rihouey, C.; Le Cerf, D.; Michaud, P.; Abdelkafi, S.; Fendri, I. Optimization of Exopolysaccharides Production by Porphyridium sordidum and Their Potential to In-duce Defense Responses in Arabidopsis thaliana against Fusarium oxysporum. Biomolecules 2021, 11, 282.
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134.
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2001, 50, 189–200.
- Burston, M.; Ranasinghe, K.; Gardi, A.; Parezanović, V.; Ajaj, R.; Sabatini, R. Design principles and digital control of advanced distributed propulsion systems. Energy 2021, 241, 122788.
- Naik, S.R.; Wala, S.M. Inflammation, Allergy and Asthma, Complex Immune Origin Diseases: Mechanisms and Therapeutic Agents. Recent Patents Inflamm. Allergy Drug Discov. 2013, 7, 62–95.
- Wei, M.; Geng, L.; Wang, Q.; Yue, Y.; Wang, J.; Wu, N.; Wang, X.; Sun, C.; Zhang, Q. Purification, characterization and immunostimulatory activity of a novel exopolysaccharide from Bacillus sp. H5. Int. J. Biol. Macromol. 2021, 189, 649–656.
- Chatterjee, S.; Mukhopadhyay, S.K.; Gauri, S.S.; Dey, S. Sphingobactan, a new alpha-mannan exopolysaccharide from Arctic Sphingobacterium sp. IITKGP-BTPF3 capable of biological response modification. Int. Immunopharmacol. 2018, 60, 84–95.
- Lin, Y.; Yang, J.; Luo, L.; Zhang, X.; Deng, S.; Chen, X.; Li, Y.; Bekhit, A.E.A.; Xu, B.; Huang, R. Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum. Mar. Drugs. 2022, 20, 332.
- Arena, A.; Maugeri, T.L.; Pavone, B.; Iannello, D.; Gugliandolo, C.; Bisignano, G. Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int. Immunopharma. Col. 2006, 6, 8–13.
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131.
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155.
- Carrión, O.; Delgado, L.; Mercade, E. New emulsifying and cryoprotective exopolysaccharide from Antarctic Pseudomonas sp. ID1. Carbohydr. Polym. 2015, 117, 1028–1034.
- Ali, P.; Shah, A.A.; Hasan, F.; Hertkorn, N.; Gonsior, M.; Sajjad, W.; Chen, F. A Glacier Bacterium Produces High Yield of Cryoprotective Exopolysaccharide. Front. Microbiol. 2020, 10, 3096.
- Bai, Y.; Zhang, P.; Chen, G.; Cao, J.; Huang, T.; Chen, K. Macrophage immunomodulatory activity of extracellular polysaccharide (PEP) of Antarctic bacterium Pseudoaltermonas sp.S-5. Int Immunopharmacol 2012, 12, 611–617.
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653.
- Bujak, M.; Frangogiannis, N.G. The role of IL-1 in the pathogenesis of heart disease. Arch. Immunol. Et Ther. Exp. 2009, 57, 165–176.
- Xing, Z.; Gauldie, J.; Cox, G.; Baumann, H.; Jordana, M.; Lei, X.F.; Achong, M.K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J. Clin. Investig. 1998, 101, 311–320.
- VanAntwerp, D.J.; Martin, S.J.; Kafri, T.; Green, D.R.; Verma, I.M. Suppression of TNF-alpha-induced apoptosis by NF-kappa B. Science 1996, 274, 787–789.
- Chen, S.; Ding, R.; Zhou, Y.; Zhang, X.; Zhu, R.; Gao, X.-D. Immunomodulatory Effects of Polysaccharide from Marine Fungus Phoma herbarumYS4108 on T Cells and Dendritic Cells. Mediat. Inflamm. 2014, 2014, 1–13.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
7 times
(View History)
Update Date:
23 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




