Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pauline Wils | -- | 1966 | 2022-08-04 15:10:43 | | | |
| 2 | Beatrix Zheng | + 5 word(s) | 1971 | 2022-08-05 05:54:34 | | | | |
| 3 | Beatrix Zheng | Meta information modification | 1971 | 2022-08-05 05:55:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wils, P.; Caron, B.; D’amico, F.; Danese, S.; Peyrin-Biroulet, L. Pain Management for Inflammatory Bowel Diseases. Encyclopedia. Available online: https://encyclopedia.pub/entry/25858 (accessed on 07 February 2026).
Wils P, Caron B, D’amico F, Danese S, Peyrin-Biroulet L. Pain Management for Inflammatory Bowel Diseases. Encyclopedia. Available at: https://encyclopedia.pub/entry/25858. Accessed February 07, 2026.
Wils, Pauline, Bénédicte Caron, Ferdinando D’amico, Silvio Danese, Laurent Peyrin-Biroulet. "Pain Management for Inflammatory Bowel Diseases" Encyclopedia, https://encyclopedia.pub/entry/25858 (accessed February 07, 2026).
Wils, P., Caron, B., D’amico, F., Danese, S., & Peyrin-Biroulet, L. (2022, August 04). Pain Management for Inflammatory Bowel Diseases. In Encyclopedia. https://encyclopedia.pub/entry/25858
Wils, Pauline, et al. "Pain Management for Inflammatory Bowel Diseases." Encyclopedia. Web. 04 August, 2022.
Copy Citation
Up to 60% of inflammatory bowel disease (IBD) patients experience abdominal pain in their lifetime regardless of disease activity. Pain negatively affects different areas of daily life and particularly impacts the quality of life of IBD patients. Despite the optimal management of intestinal inflammation, chronic abdominal pain can persist, and pharmacological and non-pharmacological approaches are necessary. Integrating psychological support in care models in IBD could decrease disease burden and health care costs. Consequently, a multidisciplinary approach similar to that used for other chronic pain conditions should be recommended.
inflammatory bowel disease
abdominal pain
quality of life
1. Overview
Many inflammatory bowel disease (IBD) therapies (anti-TNF, anti-integrin, anti-interleukin, small molecules) demonstrated their efficacy in multiple randomized controlled trials (RCTs). Nevertheless, pain improvement was not considered a key endpoint in the majority of studies even though it is an essential outcome for patients. More recently, endpoints have evolved, and abdominal pain improvement is considered a secondary endpoint in RCTs evaluating jak inhibitors (tofacitinib and upadacitinib) versus placebo in ulcerative colitis (UC) [1][2]. Control of disease activity in IBD is the first step in the management of abdominal pain, but despite an optimal management of intestinal inflammation, chronic abdominal pain can persist, and pharmacological and non-pharmacological approaches can be necessary [3][4][5][6]. Recently, the American Gastroenterological Association consensus has described the key principles in the management of functional gastrointestinal symptoms in IBD patients [7].
Figure 1 summarizes in an algorithm a sequential approach for abdominal pain management. The treatment of acute pain depends on pain intensity (assessing by VAS or NRS). Simple analgesics are often proposed in the first step, and in the case of severe pain (VAS > 55 mm or NRS > 7), opioids can be temporarily prescribed. An evaluation of IBD activity is recommended, and IBD therapies were modified accordingly. In quiescent diseases, a low FODMAPs diet may be an interesting option in IBD–IBS patients, psychological interventions may be proposed in patients with associated mood disorders, and adjuvant drugs with antidepressant and analgesic effects can be useful in most patients with chronic pain. Integrating psychological support in care models in IBD could decrease disease burden and health care costs. Consequently, a multidisciplinary approach with a mental health professional for assessing the degree of anxiety and/or depression and evaluating the probability of responding to specific medications or psychological therapies is recommended for IBD patients.
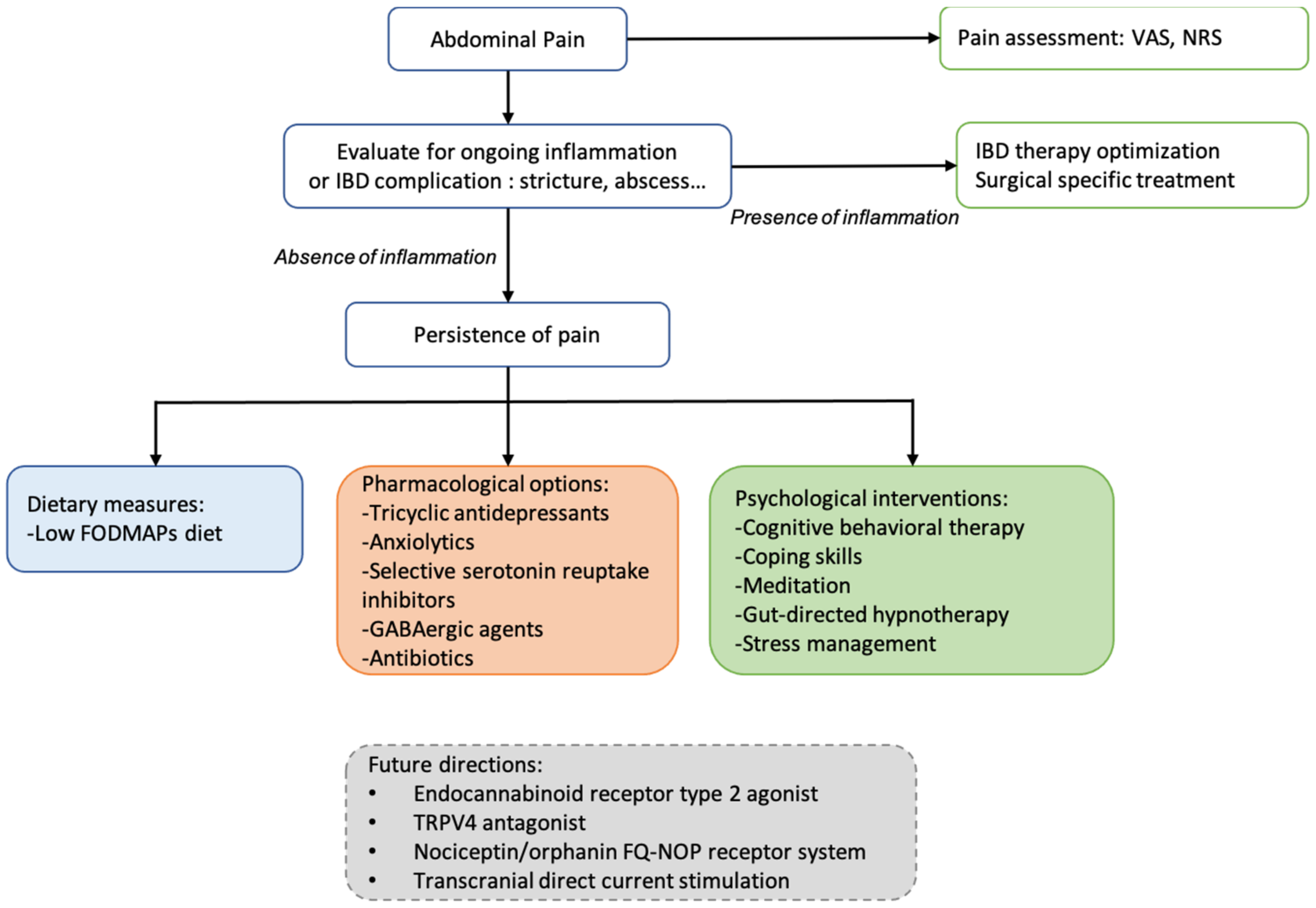
Figure 1. Proposed algorithm for pain management with pharmacological agents and non-pharmacological interventions available for improve chronic abdominal pain.
2. Current Pharmacological Options
No therapies have yet proven to be effective in managing IBD-related pain.
Nonsteroidal anti-inflammatory drugs are not recommended for long-term use because of the risk of relapse or mucosal injury. Although there is no definite evidence in IBD patients, antispasmodics are commonly used in mild pain [8]. However, antispasmodics and simple antalgics are often not sufficient, leading to the prescription of opioids. Despite their potential side effects, a high proportion of IBD patients received opioids for chronic abdominal pain [9]. Notably, in a large epidemiological study, approximately 5% of IBD patients were heavy users of opioids within 10 years of diagnosis [10]. However, the chronic use of opioids should be avoided in IBD patients due to the risks of dependence, hyperalgesia, narcotic bowel syndrome [11], and premature mortality in heavy users [7][12].
Alternative drugs to opioids should be encouraged [3][5][6][13]. Antidepressant and anxiolytic medications may be an interesting option for abdominal pain especially if there are coexisting mood disorders. In particular, a retrospective cohort study involving 81 patients with IBD revealed that tricyclic antidepressants (amitriptyline, nortriptyline, or desipramine) improved gastrointestinal symptoms (not abdominal pain specifically) [14]. An open-label preliminary study on selective serotonin reuptake inhibitors (citalopram, paroxetine, fluoxetine, and escitalopram) in psychiatric disorders suggested the efficacy of paroxetine on abdominal pain in eight IBD patients [15]. Serotonin–norepinephrine reuptake inhibitors (SNRIs) were effective for the control of chronic pain syndromes in IBS patients. Interestingly, a large Canadian cohort study of 403,665 patients with new-onset depression investigated the impacts of depression and antidepressant therapies on the development of IBD. Selective serotonin reuptake inhibitors and tricyclic antidepressants were associated with a reduced risk of developing both CD and UC, and SNRIs were protective against UC [16]. Nevertheless, these treatments are not devoid of side effects, (nausea, dry mouth, muscle spasms, constipation), bringing an additional burden to patients. Other studies suggest a potential abdominal pain-relieving effect of gabapentin and pregabalin (usually prescribed for neuropathic pain), but these have not been investigated in IBD patients [3]. Other interventions have been associated with a reduction of abdominal pain [6]. In a randomized controlled trial evaluating the efficacy of antibiotics on 29 CD patients with small intestinal bacterial overgrowth, the antibiotics metronidazole and ciprofloxacin improved abdominal pain in 50% and 43% of cases, respectively [17]. Rifaximin, a non-absorbed antibiotic, was an effective treatment of small intestinal bacterial overgrowth, improving symptoms in 67% of patients in a recent meta-analysis [18]; it could be an option for IBD patients with small intestinal bacterial overgrowth. Furthermore, restoring the microbial flora seems to be an interesting option in the management of IBS. Some probiotics have demonstrated their effectiveness in improving symptoms, including abdominal pain for patients with IBS (for example L. Plantarum 299v, B. Bifidum MIMBb75) [19][20]. The use of probiotics in IBD could be a therapeutic option to improve dysbiosis but requires further studies. Another randomized study demonstrated the efficacy of transdermal nicotine patches in 72 active UC patients versus placebo: 49% in the nicotine group had complete remission compared with 24% in the placebo group (p = 0.03), with less abdominal pain in the nicotine group (p = 0.05) [21]. A small trial evaluating loperamide oxide in CD patients with chronic diarrhea indicated an improvement in abdominal pain after one week of treatment [22]. Previous studies reported that around 10% of IBD patients use cannabis and derivatives to improve symptoms [23]. Patients developed a growing interest in the potential therapeutic effect of cannabis on IBD-related symptoms and IBD course. Data regarding the inflammatory effects of cannabis and derivatives are still limited: small studies reported an improvement of several IBD-related symptoms, including abdominal pain, but without any significant improvement of inflammation markers or disease course [24][25][26]. A phase 2a trial evaluating the efficacy and tolerability of cannabidiol in steroid-dependent CD patients is still ongoing [27]. Table 1 details pharmacological options in IBD patients with chronic abdominal pain.
Table 1. Data supporting the efficacy of pharmacological and non-pharmacological interventions in IBD patients with chronic abdominal pain.
| Treatment | Study Design | Study Intervention | Age (Year) Sex F |
Number of Patients | Abdominal Pain Outcome |
|---|---|---|---|---|---|
| Pharmacological treatment | |||||
| tricyclic antidepressants (TCA) [14] (nortriptyline, amitriptyline, desipramine, doxepin) |
Retrospective cohort study | IBD patients with inactive or mildly active disease and persistent gastrointestinal symptoms (median TCA dose: 25 mg (10–150 mg)) | 41.3 69% |
58 CD/23 UC | TCA improved gastrointestinal symptoms in 59.3% of IBD patients (Likert score ≥ 2) Response was better in UC than in CD patients (1.86 ± 0.13 vs. 1.26 ± 0.11, respectively, p = 0.003) |
| Antibiotics: metronidazole or ciprofloxacin [17] | RCT | CD patients with small intestinal bacterial overgrowth (confirmed by hydrogen/methane breath and glucose tests) receiving metronidazole 250 mg t.d.s (group A) or ciprofloxacin 500 mg b.d (group B) for 10 days | 39 41% |
29 CD | Improvement of abdominal pain in 50% (group A) and 43% (group B) of cases |
| Transdermal nicotine patch [21] | Randomized double-bind study | Transdermal nicotine (5 or 15 mg) versus placebo in active UC patients; improvement of abdominal pain was a secondary outcome. | 44 43% |
72 UC | Abdominal pain rate on 0–2 scale at 6 weeks was at 0.3 inthe nicotine group and at 0.6 in the placebo group (p = 0.05) |
| Loperamide oxide [22] | Double-blind investigation | Loperamide 1 mg or placebo after passage of each unformed stool for one week | 35 53% |
34 CD | At one week, the investigator’s assessment of the change in abdominal pain was significant for loperamide oxide (p = 0.020) but not for placebo. |
| Cannabis [24] | Monocentric cohort | Consecutive patients with IBD who had used cannabis specifically for the treatment of IBD or its symptoms were compared with those who had not | 36.6 50% (users) |
303 | 17.6% of patients used cannabis to relieve symptoms associated with their IBD. Cannabis improved abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%), and diarrhea (28.6%), although side effects were frequent. |
| Dietary measures | |||||
| Low-FODMAPs diet [28] | Retrospective telephone survey | IBD patients in remission Improvement of 5 points or more for gastrointestinal symptoms after dietary information on low-FODMAPs diet |
48 39% |
52 CD/20 UC | Approximately 70% of patients were adherent to the low-FODMAPs diet After 3 months, 56% had clinical improvement of abdominal pain (p < 0.02) |
| Psychological approaches | |||||
| Cognitive behavioral therapy [29] | RCT (CBT versus supportive nondirective therapy) | Evaluation of IBD activity (PCDAI and PUCAI) and depression in young patients (after 3-month course of CBT or supportive nondirective therapy | 14.3 46% and 52% |
161 CD and 56 UC | Compared with supportive non-directive therapy, CBT showed a greater reduction in IBD activity (p = 0.04); both psychotherapies decreased rate of depression scale |
| Gut-directed hypnotherapy [30] | RCT hypnotherapy (HPN) versus nondirective discussion | Patients received seven sessions of HPN or nondirective discussion. Evaluation of proportion of participants in each condition that had remained clinically asymptomatic through 52 weeks post treatment |
38 54% |
54 quiescent UC | 68% versus 40% of patients maintaining remission for 1 year (p = 0.04) |
| Stress management program [31] | RCT stress management, self-directed stress management, or conventional medical treatment |
CD patients considered in non-active stage of disease under sulfasalazine Evaluation of symptoms post-treatment |
31.7 64% |
45 CD | Significant decrease in abdominal pain in both stress management arms (14.2% and 6.6% versus 48%) |
Abbreviations: TCA: tricyclic antidepressants; CD, Crohn’s disease; UC, ulcerative colitis; FODMAP: fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; RCT: randomized controlled trial; CBT: cognitive behavioral therapy; PCDAI: Pediatric Crohn’s Disease Activity Index; PUCAI: Pediatric Ulcerative Colitis Activity Index; HPN: hypnotherapy.
3. Non-Pharmacological Interventions
3.1. Dietary Measures
The presence of indigestible carbohydrates or osmotic molecules in the luminal gut may result in high fermentation, the production of gas and short chain fatty acids, and distention and could participate in IBS-like symptoms such as abdominal pain in IBD patients [32]. Lactose and fructose malabsorption are frequent in CD patients, an estimated 42% and 61% of patients, respectively, in a previous study [33] and could be associated with abdominal pain. Several dietary measures including lactose-reduced or low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) diets may improve functional gastrointestinal symptoms [32]. Despite the low grade of evidence, the low-FODMAP diet proved to be effective for the IBS-like symptoms of IBD patients in remission [28]. In addition, a randomized controlled cross-over trial reported an exacerbation of functional gastrointestinal symptoms in quiescent IBD patients exposed to FODMAPs [34]. However, considering the risk of compromised nutritional status, the use of restrictive diets in IBD patients in remission should be carefully supervised by a dietician.
3.2. Psychological Approaches
Psychosocial factors impact the quality of life of IBD patients and often can benefit from non-pharmacological approaches. Notably, the consequent association between pain and depression, stress, or anxiety suggests that the optimal management of these mood disorders may improve pain levels and QOL. In the IBD field, psychological interventions, particularly cognitive behavioral therapy (CBT), have been found as valid approaches for functional gastrointestinal symptoms and pain [6][13]. Coping skill training, another psychological and educational intervention, developed to increase individuals’ ability to manage uncomfortable or anxiety-provoking situations, improved somatic symptoms in IBD adolescents but did not significantly affect pain [29]. Notably, psychological interventions were associated with clinical improvement in pediatric and adult IBD patients with anxiety or depression [30][35][36]. However, another randomized controlled trial evaluating the impact of a 10-week cognitive behavioral therapy in adult IBD patients did not find an influence of CBT in the course of IBD over 24 months [37]. Another proposed option is hypnotherapy, a technique used to create a state of focused attention and help to gain control over undesired behaviors or treat stress with suggestions for relaxation. Hypnotherapy has been associated with positive outcomes for different chronic-pain conditions (e.g., cancer, low-back pain, arthritis pain). For IBD, a prospective study demonstrated a significant effect of gut-directed hypnotherapy on prolonging clinical remission in 54 patients with quiescent UC [30]. There is also consistent evidence for a contribution of stress in the IBD disease course [38]. For this reason, a stress management program including 45 CD patients was proposed. A significant reduction in abdominal pain was detected in subjects who received training in self-directed and therapist-led stress management [31]. Acupuncture, derived from traditional Chinese medicine, is a practice designed to rebalance a patient’s Qi. In IBD patients, acupuncture can be a complementary approach that helps reduce chronic abdominal pain [39].
References
- Panés, J.; Vermeire, S.; Lindsay, J.O.; Sands, B.E.; Su, C.; Friedman, G.; Zhang, H.; Yarlas, A.; Bayliss, M.; Maher, S.; et al. Tofacitinib in Patients with Ulcerative Colitis: Health-Related Quality of Life in Phase 3 Randomised Controlled Induction and Maintenance Studies. J. Crohns Colitis 2018, 12, 145–156.
- Ghosh, S.; Sanchez Gonzalez, Y.; Zhou, W.; Clark, R.; Xie, W.; Louis, E.; Loftus, E.V.; Panes, J.; Danese, S. Upadacitinib Treatment Improves Symptoms of Bowel Urgency and Abdominal Pain, and Correlates with Quality of Life Improvements in Patients with Moderate to Severe Ulcerative Colitis. J. Crohns Colitis 2021, 15, 2022–2030.
- Srinath, A.I.; Walter, C.; Newara, M.C.; Szigethy, E.M. Pain Management in Patients with Inflammatory Bowel Disease: Insights for the Clinician. Ther. Adv. Gastroenterol. 2012, 5, 339–357.
- Regueiro, M.; Greer, J.B.; Szigethy, E. Etiology and Treatment of Pain and Psychosocial Issues in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 430–439.e4.
- Zielińska, A.; Sałaga, M.; Włodarczyk, M.; Fichna, J. Focus on Current and Future Management Possibilities in Inflammatory Bowel Disease-Related Chronic Pain. Int. J. Colorectal Dis. 2019, 34, 217–227.
- Norton, C.; Czuber-Dochan, W.; Artom, M.; Sweeney, L.; Hart, A. Systematic Review: Interventions for Abdominal Pain Management in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2017, 46, 115–125.
- Colombel, J.-F.; Shin, A.; Gibson, P.R. AGA Clinical Practice Update on Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2019, 17, 380–390.e1.
- Makharia, G.K. Understanding and Treating Abdominal Pain and Spasms in Organic Gastrointestinal Diseases: Inflammatory Bowel Disease and Biliary Diseases. J. Clin. Gastroenterol. 2011, 45, S89–S93.
- Haapamäki, J.; Tanskanen, A.; Roine, R.P.; Blom, M.; Turunen, U.; Mäntylä, J.; Färkkilä, M.A.; Arkkila, P.E.T. Medication Use among Inflammatory Bowel Disease Patients: Excessive Consumption of Antidepressants and Analgesics. Scand. J. Gastroenterol. 2013, 48, 42–50.
- Targownik, L.E.; Nugent, Z.; Singh, H.; Bugden, S.; Bernstein, C.N. The Prevalence and Predictors of Opioid Use in Inflammatory Bowel Disease: A Population-Based Analysis. Am. J. Gastroenterol. 2014, 109, 1613–1620.
- Camilleri, M.; Lembo, A.; Katzka, D.A. Opioids in Gastroenterology: Treating Adverse Effects and Creating Therapeutic Benefits. Clin. Gastroenterol. Hepatol. 2017, 15, 1338–1349.
- Burr, N.E.; Smith, C.; West, R.; Hull, M.A.; Subramanian, V. Increasing Prescription of Opiates and Mortality in Patients with Inflammatory Bowel Diseases in England. Clin. Gastroenterol. Hepatol. 2018, 16, 534–541.e6.
- Bakshi, N.; Hart, A.L.; Lee, M.C.; Williams, A.C.D.C.; Lackner, J.M.; Norton, C.; Croft, P. Chronic Pain in Patients with Inflammatory Bowel Disease. Pain 2021, 162, 2466–2471.
- Iskandar, H.N.; Cassell, B.; Kanuri, N.; Gyawali, C.P.; Gutierrez, A.; Dassopoulos, T.; Ciorba, M.A.; Sayuk, G.S. Tricyclic Antidepressants for Management of Residual Symptoms in Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2014, 48, 423–429.
- Walker, E.A.; Gelfand, M.D.; Gelfand, A.N.; Creed, F.; Katon, W.J. The Relationship of Current Psychiatric Disorder to Functional Disability and Distress in Patients with Inflammatory Bowel Disease. Gen. Hosp. Psychiatry 1996, 18, 220–229.
- Frolkis, A.D.; Vallerand, I.A.; Shaheen, A.-A.; Lowerison, M.W.; Swain, M.G.; Barnabe, C.; Patten, S.B.; Kaplan, G.G. Depression Increases the Risk of Inflammatory Bowel Disease, Which May Be Mitigated by the Use of Antidepressants in the Treatment of Depression. Gut 2019, 68, 1606–1612.
- Castiglione, F.; Rispo, A.; Di Girolamo, E.; Cozzolino, A.; Manguso, F.; Grassia, R.; Mazzacca, G. Antibiotic Treatment of Small Bowel Bacterial Overgrowth in Patients with Crohn’s Disease. Aliment. Pharmacol. Ther. 2003, 18, 1107–1112.
- Gatta, L.; Scarpignato, C. Systematic Review with Meta-Analysis: Rifaximin Is Effective and Safe for the Treatment of Small Intestine Bacterial Overgrowth. Aliment. Pharmacol. Ther. 2017, 45, 604–616.
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical Trial: Lactobacillus Plantarum 299v (DSM 9843) Improves Symptoms of Irritable Bowel Syndrome. World J. Gastroenterol. 2012, 18, 4012–4018.
- Guglielmetti, S.; Mora, D.; Gschwender, M.; Popp, K. Randomised Clinical Trial: Bifidobacterium Bifidum MIMBb75 Significantly Alleviates Irritable Bowel Syndrome and Improves Quality of Life—A Double-Blind, Placebo-Controlled Study. Aliment. Pharmacol. Ther. 2011, 33, 1123–1132.
- Pullan, R.D.; Rhodes, J.; Ganesh, S.; Mani, V.; Morris, J.S.; Williams, G.T.; Newcombe, R.G.; Russell, M.A.; Feyerabend, C.; Thomas, G.A. Transdermal Nicotine for Active Ulcerative Colitis. N. Engl. J. Med. 1994, 330, 811–815.
- Van Outryve, M.; Toussaint, J. Loperamide Oxide for the Treatment of Chronic Diarrhoea in Crohn’s Disease. J. Int. Med. Res. 1995, 23, 335–341.
- Swaminath, A.; Berlin, E.P.; Cheifetz, A.; Hoffenberg, E.; Kinnucan, J.; Wingate, L.; Buchanan, S.; Zmeter, N.; Rubin, D.T. The Role of Cannabis in the Management of Inflammatory Bowel Disease: A Review of Clinical, Scientific, and Regulatory Information. Inflamm. Bowel Dis. 2019, 25, 427–435.
- Storr, M.; Devlin, S.; Kaplan, G.G.; Panaccione, R.; Andrews, C.N. Cannabis Use Provides Symptom Relief in Patients with Inflammatory Bowel Disease but Is Associated with Worse Disease Prognosis in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2014, 20, 472–480.
- Naftali, T.; Bar-Lev Schleider, L.; Dotan, I.; Lansky, E.P.; Sklerovsky Benjaminov, F.; Konikoff, F.M. Cannabis Induces a Clinical Response in Patients with Crohn’s Disease: A Prospective Placebo-Controlled Study. Clin. Gastroenterol. Hepatol. 2013, 11, 1276–1280.e1.
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Pilot Study of Cannabidiol-Rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724.
- Stero Biotechs Ltd. A Phase 2a Study to Evaluate the Safety, Tolerability and Efficacy of Cannabidiol as a Steroid-Sparing Therapy in Steroid-Dependent Crohn’s Disease Patients. Available online: https://ClinicalTrials.gov/show/NCT04056442 (accessed on 12 July 2022).
- Gibson, P.R. Use of the Low-FODMAP Diet in Inflammatory Bowel Disease. J. Gastroenterol. Hepatol. 2017, 32, 40–42.
- McCormick, M.; Reed-Knight, B.; Lewis, J.D.; Gold, B.D.; Blount, R.L. Coping Skills for Reducing Pain and Somatic Symptoms in Adolescents with IBD. Inflamm. Bowel Dis. 2010, 16, 2148–2157.
- Keefer, L.; Taft, T.H.; Kiebles, J.L.; Martinovich, Z.; Barrett, T.A.; Palsson, O.S. Gut-Directed Hypnotherapy Significantly Augments Clinical Remission in Quiescent Ulcerative Colitis. Aliment. Pharmacol. Ther. 2013, 38, 761–771.
- García-Vega, E.; Fernandez-Rodriguez, C. A Stress Management Programme for Crohn’s Disease. Behav. Res. Ther. 2004, 42, 367–383.
- Camilleri, M.; Boeckxstaens, G. Dietary and Pharmacological Treatment of Abdominal Pain in IBS. Gut 2017, 66, 966–974.
- Barrett, J.S.; Irving, P.M.; Shepherd, S.J.; Muir, J.G.; Gibson, P.R. Comparison of the Prevalence of Fructose and Lactose Malabsorption across Chronic Intestinal Disorders. Aliment. Pharmacol. Ther. 2009, 30, 165–174.
- Cox, S.R.; Prince, A.C.; Myers, C.E.; Irving, P.M.; Lindsay, J.O.; Lomer, M.C.; Whelan, K. Fermentable Carbohydrates Exacerbate Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease: A Randomised, Double-Blind, Placebo-Controlled, Cross-over, Re-Challenge Trial. J. Crohns Colitis 2017, 11, 1420–1429.
- Szigethy, E.; Bujoreanu, S.I.; Youk, A.O.; Weisz, J.; Benhayon, D.; Fairclough, D.; Ducharme, P.; Gonzalez-Heydrich, J.; Keljo, D.; Srinath, A.; et al. Randomized Efficacy Trial of Two Psychotherapies for Depression in Youth with Inflammatory Bowel Disease. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 726–735.
- Gerbarg, P.L.; Jacob, V.E.; Stevens, L.; Bosworth, B.P.; Chabouni, F.; DeFilippis, E.M.; Warren, R.; Trivellas, M.; Patel, P.V.; Webb, C.D.; et al. The Effect of Breathing, Movement, and Meditation on Psychological and Physical Symptoms and Inflammatory Biomarkers in Inflammatory Bowel Disease: A Randomized Controlled Trial. Inflamm. Bowel Dis. 2015, 21, 2886–2896.
- Mikocka-Walus, A.; Bampton, P.; Hetzel, D.; Hughes, P.; Esterman, A.; Andrews, J.M. Cognitive-Behavioural Therapy for Inflammatory Bowel Disease: 24-Month Data from a Randomised Controlled Trial. Int. J. Behav. Med. 2017, 24, 127–135.
- Maunder, R.G.; Levenstein, S. The Role of Stress in the Development and Clinical Course of Inflammatory Bowel Disease: Epidemiological Evidence. Curr. Mol. Med. 2008, 8, 247–252.
- Berger, A.A.; Liu, Y.; Jin, K.; Kaneb, A.; Welschmeyer, A.; Cornett, E.M.; Kaye, A.D.; Imani, F.; Khademi, S.-H.; Varrassi, G.; et al. Efficacy of Acupuncture in the Treatment of Chronic Abdominal Pain. Anesth. Pain Med. 2021, 11, e113027.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Gastrointestinal Disease
Revisions:
3 times
(View History)
Update Date:
05 Aug 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




