Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeff Myers | -- | 3257 | 2022-07-19 19:55:16 | | | |
| 2 | Sirius Huang | -20 word(s) | 3237 | 2022-07-20 03:33:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gorensek-Benitez, A.H.; Kirk, B.; Myers, J.K. Synthetic Polymers and Protein Fibrillation under Crowded Conditions. Encyclopedia. Available online: https://encyclopedia.pub/entry/25292 (accessed on 08 February 2026).
Gorensek-Benitez AH, Kirk B, Myers JK. Synthetic Polymers and Protein Fibrillation under Crowded Conditions. Encyclopedia. Available at: https://encyclopedia.pub/entry/25292. Accessed February 08, 2026.
Gorensek-Benitez, Annelise H., Bryan Kirk, Jeffrey K. Myers. "Synthetic Polymers and Protein Fibrillation under Crowded Conditions" Encyclopedia, https://encyclopedia.pub/entry/25292 (accessed February 08, 2026).
Gorensek-Benitez, A.H., Kirk, B., & Myers, J.K. (2022, July 19). Synthetic Polymers and Protein Fibrillation under Crowded Conditions. In Encyclopedia. https://encyclopedia.pub/entry/25292
Gorensek-Benitez, Annelise H., et al. "Synthetic Polymers and Protein Fibrillation under Crowded Conditions." Encyclopedia. Web. 19 July, 2022.
Copy Citation
Protein amyloid fibrils have widespread implications for human health. Fibrillation has been studied using a variety of crowding agents to mimic the packed interior of cells or to probe the mechanisms and pathways of the process. One commonly used class of crowding agents is synthetic polymers. Complex effects are observed depending on the specific paring of polymer and fibrillating protein, but generally crowding with synthetic polymers favors fibrillation.
amyloid fibril
excluded volume
molecular crowding
protein fibrillation
synthetic polymer
viscosity
1. Synthetic Polymers as Crowding Agents
Synthetic polymers, including the sugar-based polymers Ficoll and dextran [1][2][3][4], polyethylene glycol (PEG) [5], and polyvinylpyrrolidone (PVP) [6][7], are commonly used to represent the crowded cellular environment (blue reference numbers refer to the citation list given in Goresek-Benitez et al [1]. These polymers affect the proteins via both steric repulsion and weak chemical interactions. The effects of their monomers, some of which are osmolytes [8], can be used to contextualize and decode the effects of the polymers [2][3][9]. The bond-line structures of commonly used synthetic polymers are presented in Figure 1.
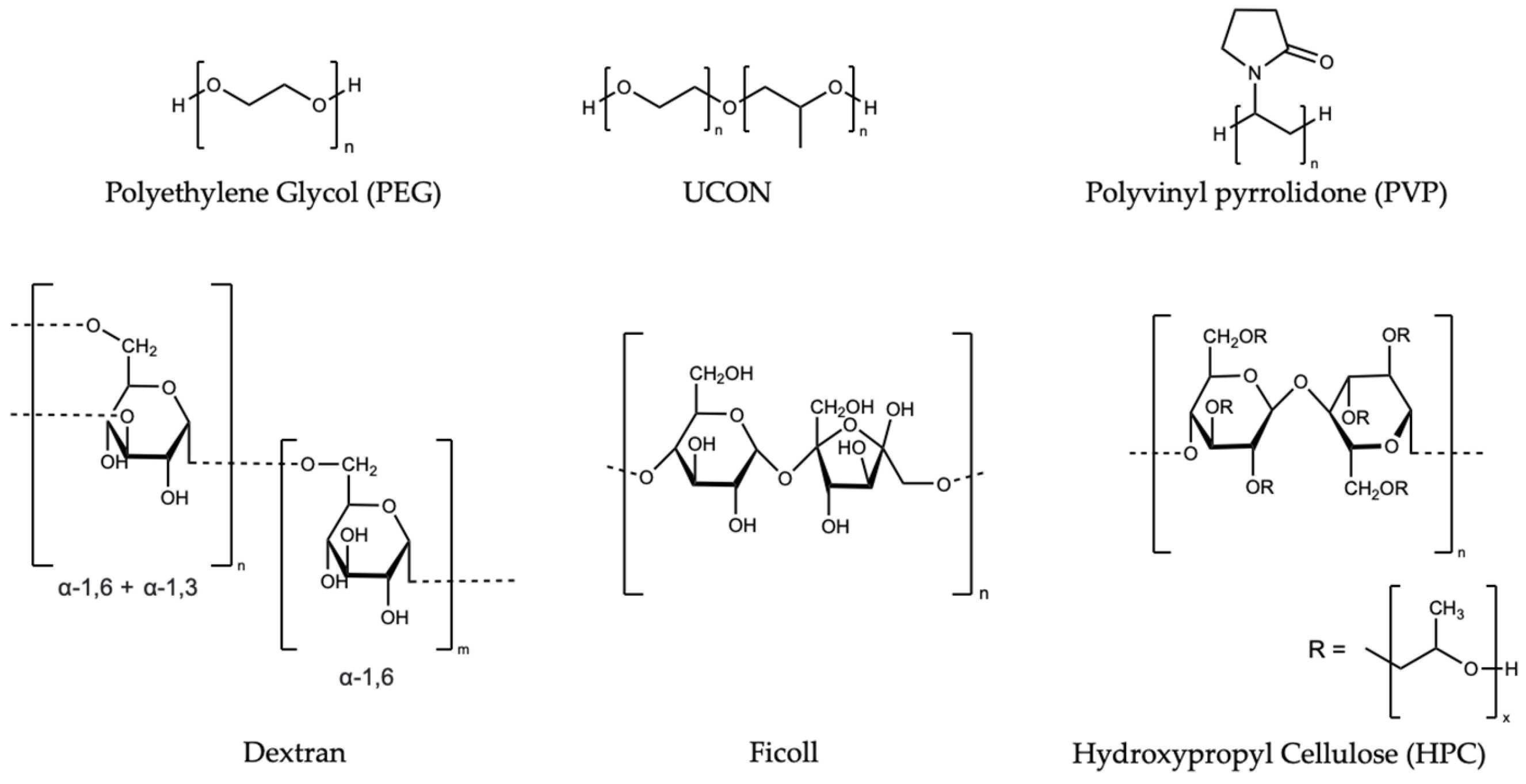
Figure 1. Structures of synthetic polymers referenced in the following section.
Ultimately, the synthetic polymers are not the best representation of cells [9][10]. Another option is to use reconstituted cytosol, lysates [10][11][12] or model proteins, such as hen egg white lysozyme (HEWL or lysozyme), and bovine serum albumin (BSA) [2][13] as the crowders. However, these biopolymers still fall short of accurately replicating the cellular interior [2]. Both synthetic and physiologically relevant crowders pose challenges not seen in dilute solution experiments, including increased solution viscosity, high background, and decreased signal quality due to interactions between crowders and test proteins [3][4]. The effects of crowding on protein structure and function have been probed in living cells, but in-cell experiments pose many of the same challenges, with the additional concern of cell leakage [14][15][16][17][18][19].
2. Synthetic Polymers and Protein Fibrillation
References and results from fibrillation experiments under crowded solutions are listed in Table 1.
Table 1. Effects of synthetic polymers on protein fibrillation.
| Polymer | Test Protein | Effect |
|---|---|---|
| Ficoll 70 | α-synuclein | Promotes fibrillation [5][13][20][21] |
| Tau Protein | Promotes fibrillation [22] | |
| Insulin, pH = 2 | Promotes fibrillation [20] | |
| Insulin, pH = 7.5 | Hinders fibrillation [20] | |
| Bovine Carbonic Anhydrase | Hinders fibrillation [23] | |
| hIAPP | No effect [24] | |
| Unphosphorylated Tau 244–372 | Promotes Fibrillation [25] | |
| Phosphorylated Tau 244–441 | Promotes Fibrillation [25] | |
| Human PrP | Promotes Fibrillation [25] | |
| Human PrP E196K | Promotes Fibrillation [25] | |
| Human PrP D178N | Promotes Fibrillation [25] | |
| Rabbit PrP | Hinders Fibrillation [25] | |
| HEWL | Hinders Fibrillation [25] | |
| α-lactalbumin | Promotes Fibrillation [20] | |
| Ficoll 400 | α-synuclein | Promotes fibrillation [5][13][20] |
| Human PrP | Promotes Fibrillation [25] | |
| Human PrP E196K | Promotes Fibrillation [25] | |
| Human PrP D178N | Promotes Fibrillation [25] | |
| Dextran 70 | Human Tau protein, 50–100 g/L | Promotes fibrillation [22] |
| Human Tau protein, 150 g/L | Hinders fibrillation [22] | |
| α-synuclein | Promotes fibrillation [21] | |
| Bovine Carbonic Anhydrase | Hinders fibrillation [23] | |
| hIAPP, 10–20% | No effect [24] | |
| hIAPP, 30–40% | Hinders fibrillation [24] | |
| Hemoglobin | Promotes fibrillation [26] | |
| Unphosphorylated Tau 244–372 | Promotes fibrillation [25] | |
| Phosphorylated Tau 244–441 | Promotes fibrillation [25] | |
| SOD1 A4V | Promotes fibrillation [25] | |
| Rabbit PrP | Hinders fibrillation [25] | |
| HEWL | Hinders fibrillation [25] | |
| β-lactoglobulin | Promotes fibrillation [27] | |
| Dextran 100 | α-lactalbumin | Increases lag phase, decreases elongation rate [28] |
| Insulin, pH = 2.5 | Decreases elongation rate [28] | |
| Insulin, pH = 7.5 | Increases lag phase, decreases elongation rate [28] | |
| HEWL | Decreases elongation rate [28] | |
| α-synuclein | Decreases lag phase and elongation rate [28] | |
| Histone | Decreases lag phase [28] | |
| Dextran 138 | α-synuclein | Promotes fibrillation [5][13] |
| Dextran 200 | HEWL | Promotes fibrillation [29] |
| Insulin | Promotes fibrillation [29] | |
| PI3-SH3 | Promotes fibrillation [29] | |
| α-synuclein | Promotes fibrillation [29] | |
| Insulin β Chain | Promotes fibrillation [29] | |
| Dextran 250 | Insulin, pH = 2.5 | Decreases elongation rate [28] |
| Insulin, pH = 7.5 | Increases lag phase, decreases elongation rate [28] | |
| HEWL | Decreases elongation rate [28] | |
| α-synuclein | Decreases lag phase and elongation rate [28] | |
| Histone | Decreases lag phase, increases elongation rate [28] | |
| α-lactalbumin | Increases lag phase, decreases elongation rate [28] | |
| Dextran 500 | Insulin, pH = 2.5 | Decreases elongation rate [28] |
| Insulin, pH = 7.5 | Increases lag phase, decreases elongation rate [28] | |
| HEWL | Decreases elongation rate [28] | |
| α-synuclein | Decreases lag phase and elongation rate [28] | |
| Histone | Decreases lag phase, increases elongation rate [28] | |
| α-lactalbumin | Increases lag phase, decreases elongation rate [28] | |
| PEG 200 | α-synuclein | Promotes fibrillation [5][13] |
| PEG 400 | β-lactoglobulin | Promotes fibrillation [27] |
| α-synuclein | Promotes fibrillation [5][13] | |
| PEG 600 | α-synuclein | Promotes fibrillation [5][13], Hinders fibrillation [30] |
| PEG 1000 | α-synuclein | Increases lag time and fibrillation rate [30] |
| PEG 3350 | α-synuclein | Promotes fibrillation [5] |
| PEG 3500 | Insulin pH = 2 | Promotes fibrillation [20] |
| Insulin pH = 7.5 | Hinders fibrillation [20] | |
| Histones pH = 2.5 | Promotes fibrillation [20] | |
| Histones pH = 7.5 | Hinders fibrillation [20] | |
| α-synuclein | Promotes fibrillation [5][20][21] | |
| α-lactalbumin | Promotes fibrillation [20] | |
| PEG 4000 | α-synuclein | Decreases lag time and fibrillation rate [30] |
| Hemoglobin | Promotes fibrillation [26] | |
| PEG 4400 | α-synuclein | Promotes fibrillation [31] |
| Insulin | Promotes fibrillation [31] | |
| PEG 6000 | Hemoglobin | Promotes fibrillation [26] |
| PEG 8000 | β-lactoglobulin | Promotes fibrillation [27]. |
| PEG 10,000 | α-synuclein | Promotes fibrillation [5] |
| PEG 20,000 | HEWL, unseeded | Hinders fibrillation [32] |
| HEWL, seeded | Promotes fibrillation [32] | |
| Unphosphorylated Tau 244–372 | Promotes fibrillation [25] | |
| SOD1 A4V | Promotes fibrillation [25] | |
| Rabbit PrP | Hinders fibrillation [25] | |
| β-lactoglobulin | Promotes fibrillation [27] | |
| PEG 200,000 | Insulin | Promotes Fibrillation [29] |
| HPC 100 | Insulin pH = 2.5 | Increases lag time and decreases elongation rate [28] |
| Insulin pH = 7.5 | Increases lag time and decreases elongation rate [28] | |
| α-synuclein | Increases lag time and decreases elongation rate [28] | |
| α-lactalbumin | Increases lag time and decreases elongation rate [28] | |
| HPC 370 | Insulin pH = 2.5 | Increases lag time and decreases elongation rate [28] |
| Insulin pH = 7.5 | Increases lag time and decreases elongation rate [28] | |
| α-synuclein | Increases lag time and decreases elongation rate [28] | |
| α-lactalbumin | Increases lag time and decreases elongation rate [28] | |
| HPC 1000 | Insulin pH = 2.5 | Increases lag time and decreases elongation rate [28] |
| Insulin pH = 7.5 | Increases lag time and decreases elongation rate [28] | |
| α-synuclein | Increases lag time and decreases elongation rate [28] | |
| α-lactalbumin | Increases lag time and decreases elongation rate [28] | |
| UCON 5400 | α-synuclein | Hinders fibrillation [31] |
| Insulin | Hinders fibrillation [31] |
In their 2010 Journal of the American Chemical Society publication [29], Dobson and coworkers studied the effects of the synthetic PEG 200,000, dextran 200, and the dextran monomer and osmolyte, glucose. The study used pre-nucleated fibrils, enabling measurements that exclusively probe the elongation rates. They interpreted their results using the framework of scaled-particle theory [33][34][35], which posits that the excluded volume effects decrease with the increasing particle size. The limits of scaled particle theory to analyze the crowding effects on fibrillation were acknowledged, as the parameters of the study can only account for the fibril elongation. As expected, the analysis of fibrillation in the complex cellular matrix has seen limited success [36]. The effects of PEG 200,000 and dextran 200 are considered here.
A variety of amyloid-prone proteins of different sizes were used, because scaled particle theory predicts that the fibrillation rates increase with the increasing hydrodynamic radius of the precursor protein. The proteins include the globular proteins, lysozyme and insulin, and proteins lacking a well-defined tertiary structure, including the SH3 domain of the phosphatidyl-inositase-3-kinase (SH3), α-synuclein, and the β-domain of insulin at pH 2. The amyloid elongation was measured as a function of the increasing dextran 200 ranging from 0–60 g/L. Dextran 200 accelerates the relative fibrillation elongation rates of all of the proteins, and this enhancement increases with the increasing hydrodynamic radius of the test protein. As the trends with an increasing extent of acceleration as a function of the protein hydrodynamic radius is consistent with scaled particle theory, these affects are attributed to the volume exclusion by dextran 200. PEG 200,000 was also found to accelerate the relative rate of elongation of insulin, and to a greater extent than dextran 200. The promotion of fibrillation in the synthetic polymers is also observed in several other studies [5][13][20][21].
These findings agree with the pioneering work of Uversky and coworkers, which began with synthetic polymers and α-synuclein, the protein implicated in Parkinson’s Disease [13]. The crowders’ identity, size, and concentration were considered. PEG, Ficoll, and dextran promote fibrillation by increasing the rate and decreasing the lag time. Of the three types of polymers, PEG is the most drastic accelerant. The PEGs with the largest molecular weight (3350 Da) exert stronger effects than the smaller PEGs (200 Da, 400 Da, 600 Da). The fibrillation is increasingly accelerated as the PEG 3350 concentration increases from 25 to 150 mg/mL.
While PEG most effectively promotes α-synuclein fibrillation, the effects of dextran 138, Ficoll 70, and Ficoll 400 were also considered. Ficoll 400 is slightly more effective than Ficoll 70 at increasing the fibrillation rate and decreasing the lag time, but both are more effective than dextran 138. Ultimately, the authors observe the modulation of the fibrillation depends on the identity of the polymer, and within a single type of polymer, the fibrillation increases with increasing size and concentration. The authors attribute these effects to excluded volume, and eliminated increased solution viscosity as an explanation, as polymers decreased the lag time of the reaction.
A subsequent publication expanded the exploration to a variety of proteins, including S-carboxymethyl lactalbumin, human insulin, bovine core histones, and human α-synuclein [20]. Whereas the proteins selected by Dobson and coworkers vary in degrees of disorder, these proteins additionally vary in oligomeric state. Consistent with other studies of α-synuclein [13][29], the polymers such as Ficoll 70 and PEG 3500 accelerate the fibrillation of disordered proteins, namely α-synuclein and S-carboxymethyl lactalbumin, by increasing the fibrillation rate and decreasing the lag time. The proteins that occupy an oligomeric state before fibrillation, such as bovine core histones, see hindered fibrillation in the presence of PEG 3500. Another example, human insulin, illustrates the complexity of crowding effects, as it can adopt both a monomeric and hexameric state under experimental conditions. The observations for monomeric insulin in the presence of PEG 3500 and Ficoll 70 are consistent with observations for α-synuclein and S-carboxymethyl lactalbumin at a neutral pH; where insulin is a hexamer the polymers slow fibrillation, increasing the lag time, because the oligomer must first dissociate and undergo a structural change. For the oligomeric proteins, therefore, the polymer crowders hinder fibrillation, probably because the crowded conditions favor the formation of the native oligomer.
In subsequent publications, the Uversky group probed the role of polymer morphology and flexibility [28]. The authors asserted that the effect of crowding depends on a test protein’s shape, size, and degree of order. The commonly-used synthetic polymers, dextran and Ficoll, are compact and flexible polysaccharides. However, most biopolymers in the cell—nucleic acids, proteins, etc.—are more rigid. The cellulose-derived polymers, hydroxypropyl cellulose (HPC) 100, 370, and 1000 were chosen to represent the effects of the more rigid polymers, while the dextrans 100, 250, and 500 were used to represent the more commonly used flexible polymers (See Figure 1 for a structural comparison). Unsurprisingly, the two types of polymers exhibit opposite effects on the proteins with different characteristics. The dextrans inhibit the proteins that form stable oligomers before or during fibrillation, including insulin at pH 7.5 and α-lactalbumin. By contrast, the dextrans accelerate the fibrillation of the disordered proteins, α-synuclein and histones. Modest effects in either direction are seen with the monomeric globular proteins, lysozyme and insulin, at pH 2.5. This trend indicates that the dextrans operate by excluded volume, favoring the most compact form of the test protein. The HPCs of all sizes, on the other hand, hindered fibrillation for all of the proteins—with the exception of histones, which may be due to the inability of histones to fold under the assay conditions.
Of particular interest were the contributions from excluded volume, viscosity, and weak interactions (such as electrostatic interactions, dipole–dipole interactions, and hydrogen bonds). To parse the effects of the excluded volume and viscosity, dextran 500 and Ficoll 400 (which has a similar size but a higher density and lower viscosity) were used as the crowders. These two polymers should exert roughly the same excluded volume, based on their close average molecular weight. Both dextran 500 and Ficoll 400 also hindered α synuclein and monomeric insulin fibrillation. However, Ficoll 400 did so more effectively, indicating that the excluded volume effects of dextran are likely counteracted by viscosity. However, an inhibition of fibrillation was still seen in the solutions of relatively low viscosity, suggesting the contribution of weak chemical interactions between the proteins and polymers [28].
Next, the role of polymer hydrophobicity was probed [31]. Most of the commonly-used synthetic polymers are hydrophilic. UCON 5400 (1:1 copolymer of ethylene- and propylene- glycol, Figure 1) is structurally similar to PEG 4400 but has an extra methyl group on every other unit. The additional methyl group on this polymer, in contrast to PEG, provides an excellent comparison of hydrophobicity. The effects on the secondary structure and intrinsic fluorescence quenching for 10 proteins of varying size, degree of structure, and oligomeric state were probed, while the fibrillation kinetics and morphology were explored. Circular Dichroism (CD), 8-anilonapthalene-1-sulfonate (ANS) fluorescence, and acrylamide quenching demonstrate that, while PEG and UCON do not affect the protein secondary structure, they change the solvent accessibility. Ultimately, UCON is more effective at unfolding the test protein than PEG. As with previous studies, PEG enhances the fibrillation of α-synuclein and monomeric insulin, decreasing the lag time and increasing the elongation rate. UCON, however, inhibits the fibrillation of insulin, and further analysis of the samples with a scanning electron microscopy (SEM) revealed UCON instead promotes the oligomerization of α-synuclein. Uversky and colleagues attributed the PEG effects to excluded volume, while the UCON effects were suggested to arise from changes in the solvent properties.
The enhanced fibrillation of α-synuclein and other disordered proteins in synthetic polymers, as seen in Uversky’s early studies [5][13][37] and Dobson’s publication [29], is observed in other instances. Shtilerman and colleagues observed size-dependent reduction of lag times; PEG 3350 exerts the most dramatic effect, followed by dextran 70 and Ficoll 70, while a similar trend was observed with PEGs of varying sizes. [21]. In another study, β-lactoglobulin fibrillation is accelerated—specifically the lag time decreases and the fibrillation rate increases—in Ficoll 70 and PEG 400 (400 Da), 8000 (8000 Da), and 20,000 (2000 Da). The effect is more pronounced with an increasing size and concentration, and therefore is attributed to excluded volume [27]. Wu and colleagues observed that Ficoll 70 and dextran 70 enhanced the fibrillation of human Tau protein, with dextran 70 exerting stronger effects [22].
A fibrillation-prone fragment of Tau protein was the subject of a study by Ma et al. [37], who also examined a cohort of other pathogenic, fibrillation-prone proteins, including human prion protein (PrP), and its variants E196K and D178N, the A4V SOD1 which is implicated in ALS [25]. In addition, rabbit PrP and hen egg white lysozyme were considered, both of which are not pathogenic. Ficoll 70, dextran 70, and PEG 2000 promote the fibrillation of both the unphosphorylated versions of the Tau fragment, from 50 g/L to 200 g/L. Similarly, crowding with Ficoll 70 and Ficoll 400 accelerates the fibrillation of the human prion protein, and variant, while dextran 70 and PEG 2000 enhance the SOD1 fibrillation.
The authors observed that the phosphorylated Tau protein fragment, which is associated with the onset of Alzheimer’s Disease, does not fibrillate in dilute solution. However, it fibrillates in the presence of Ficoll 70 and dextran 70, which the authors attribute to one of two explanations. The first possibility is that the phosphorylated Tau is more likely to fibrillate in a crowded environment, while the second is that crowding works to counteract the retardation initiated by phosphorylation.
Conversely, the authors found that the macromolecular crowding promote the fibrillation of the non-fibrillation-prone proteins, rabbit PrP and hen egg white lysozyme, at 100 g/L but hindered the fibrillation at 200 and 300 g/L. Whereas Dobson and coworkers saw an acceleration in the presence of crowders, regardless of the protein’s structure; thus, these authors concluded that the macromolecular crowding effects vary depending on the protein and crowder selected. Proteins that are prone to fibrillate will do so under crowded, cell-like conditions, as crowding stabilizes the aggregates or multimers along the path to aggregation. For proteins that are not aggregation-prone, such as lysozyme and rabbit Prp, the authors hypothesized that competition between the stabilization of aggregates and of the folded, native state, come into play, which led to the disparate results at 100, 200, and 300/gL crowder.
A recent study by Biswas and coworkers [30] demonstrated that the polymers of differing sizes can have opposing effects on the α-synuclein fibrillation. Specifically, with in vitro experiments, the lowest molecular weight PEG, PEG 600, hindered fibrillation by increasing the lag time. PEG 1000 increased the lag time, and the fibrillation rate, but the increase in lag time was more dramatic, hindering fibrillation. The higher-mass PEGs, PEG 4000 and PEG 12,000, both led to decreases in the lag time and in the fibrillation rate, with a more drastic reduction seen with PEG 20000. For the larger PEGs, the decrease in lag time was more dramatic, therefore promoting fibrillation. Overall, the promotion of fibrillation by the higher molecular weight PEGS was consistent with the findings of Dobson and coworkers [29], as well as other earlier studies [5][13]. Next, the authors explored how the presence of PEG 6400 and 8000 affected the fibrillation of the α-synuclein A53T in yeast cells; they found that the effect was opposite of that found in vitro. In living cells, the addition of these two PEGs hindered fibrillation. However, the authors found that the concentration of soluble α-synuclein in the cells was higher with the PEGs added than without, suggesting that in the cells, PEG may be acting to solubilize the α-synuclein monomers, and therefore working to counter aggregation.
The globular protein hemoglobin was the subject of a thorough study by Siddiqui and Naeem [26]. Specifically, the authors investigated the effects of PEG 4000, PEG 6000, and dextran 70 on hemoglobin fibrillation, and then used isothermal titration microcalorimetry (ITC) to quantify any weak interactions between the protein and the polymers. Hemoglobin, on its own under the conditions selected in the paper, did not undergo fibrillation. PEG 4000, 6000, and dextran 70, at a concentration of 200 g/L, were all found to promote hemoglobin fibrillation, with dextran 70 exhibiting the strongest effects. From the ITC experiments, it was determined that the specific binding of hemoglobin by polymers was not responsible for this change. The characteristics of the hemoglobin fibrils, however, were shown to be different, depending on the crowder. The fibrillation experiments with Congo Red demonstrated that PEG 4000 promotes the formation of protofibirils, while the fibrils were formed in the presence of PEG 6000 and dextran 70, the results of which were confirmed using morphological studies of hemoglobin aggregates using SEM. The authors then expanded upon the study by determining the effects of such aggregation on living cells, specifically, of human peripheral blood cells (PBMCs). The formation of hemoglobin aggregates in the presence of crowders reduced cell viability, manifested by an increase in lipid peroxidation, a decrease in mitochondrial membrane potential, and an increase in the number of necrotic cells relative to apoptotic cells. Additionally, the cells with aggregating hemoglobin in the presence of crowders showed increased DNA damage. All of these effects were attributed to an increase in the radical oxygen species from oxidative stress. Ultimately, the increase in the protein fibrillation due to the presence of the macromolecular crowding was demonstrated to have potentially devastating physiological impacts. This is of particular concern to the elderly, where proteopathies occur more frequently, as the cells shrink and dehydrate with age.
Dobson’s work, and the work of others, show that crowding by synthetic polymers generally accelerates fibrillation. However, several studies report mixed results: polymers either hinder the fibrillation, or have no effect. For example, Ficoll 70 and dextran 70 stabilize the native state of the β-sheet rich protein, bovine carbonic anhydrase, leading to decreased fibrillation rates and fewer aggregates [23]. Kong and Zeng found that the effects on lysozyme fibrillation depend on whether the fibrils are pre-seeded [32]. When the lysozyme fibrils are not pre-seeded, adding PEG slows the fibrillation. This trend is reversed when the lysozyme is pre-seeded, with PEG accelerating the fibrillation, suggesting that crowding stabilizes the intermediate oligomers instead of the fibrils, while the pre-formation of anchors for fibrils encourages fibrillation. The authors saw the effects on lysozyme fibrillation, even at low PEG concentrations of 10–20 g/L. The presence of the effects at such small concentrations of polymer crowders led the authors to conclude that the chemical interactions are a key factor.
The Winter group assessed the effects of a variety of crowding agents on human islet amyloid polypeptide (hIAPP), which is implicated in Type 2 Diabetes. Contrary to the other crowding agents in the study, Ficoll 70 does not affect the fibrillation of hIAPP. Low concentrations of dextran 70 (10–20%) also do not affect the fibrillation, while high concentrations of dextran (30–40%) caused a loss in the sigmoidal shape of the data and a lengthened elongation time, which the authors suggest is the result of a more complex fibrillation mechanism. The slight differences in these effects were attributed to viscosity, as dextran exhibits a higher viscosity than Ficoll [24].
References
- Christiansen, A.; Wittung-Stafshede, P. Quantification of Excluded Volume Effects on the Folding Landscape of Pseudomonas Aeruginosa Apoazurin In Vitro. Biophys. J. 2013, 105, 1689–1699.
- Smith, A.E.; Zhou, L.Z.; Gorensek, A.H.; Senske, M.; Pielak, G.J. In-Cell Thermodynamics and a New Role for Protein Surfaces. Proc. Natl. Acad. Sci. USA 2016, 113, 1725–1730.
- Gorensek-Benitez, A.H.; Smith, A.E.; Stadmiller, S.S.; Perez Goncalves, G.M.; Pielak, G.J. Cosolutes, Crowding, and Protein Folding Kinetics. J. Phys. Chem. B 2017, 121, 6527–6537.
- Acosta, L.C.; Perez Goncalves, G.M.; Pielak, G.J.; Gorensek-Benitez, A.H. Large Cosolutes, Small Cosolutes, and Dihydrofolate Reductase Activity. Protein Sci. 2017, 26, 2417–2425.
- Munishkina, L.A.; Cooper, E.M.; Uversky, V.N.; Fink, A.L. The Effect of Macromolecular Crowding on Protein Aggregation and Amyloid Fibril Formation. J. Mol. Recognit. 2004, 17, 456–464.
- Charlton, L.M.; Barnes, C.O.; Li, C.; Orans, J.; Young, G.B.; Pielak, G.J. Residue-Level Interrogation of Macromolecular Crowding Effects on Protein Stability. J. Am. Chem. Soc. 2008, 130, 6826–6830.
- Li, C.; Wang, Y.; Pielak, G.J. Translational and Rotational Diffusion of a Small Globular Protein under Crowded Conditions. J. Phys. Chem. B 2009, 113, 13390–13392.
- Somero, G.N. Protons, Osmolytes, and Fitness of Internal Milieu for Protein Function. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1986, 251, R197–R213.
- Benton, L.A.; Smith, A.E.; Young, G.B.; Pielak, G.J. Unexpected Effects of Macromolecular Crowding on Protein Stability. Biochemistry 2012, 51, 9773–9775.
- Sarkar, M.; Lu, J.; Pielak, G.J. Protein Crowder Charge and Protein Stability. Biochemistry 2014, 53, 1601–1606.
- Welte, H.; Kovermann, M. Insights into Protein Stability in Cell Lysate by 19F NMR Spectroscopy. ChemBioChem 2020, 21, 3575–3579.
- Latham, M.P.; Kay, L.E. Is Buffer a Good Proxy for a Crowded Cell-Like Environment? A Comparative NMR Study of Calmodulin Side-Chain Dynamics in Buffer and E. Coli Lysate. PLoS ONE 2012, 7, e48226.
- Uversky, V.N.; Cooper, E.M.; Bower, K.S.; Li, J.; Fink, A.L. Accelerated α-Synuclein Fibrillation in Crowded Milieu. FEBS Lett. 2001, 515, 99–103.
- Monteith, W.B.; Pielak, G.J. Residue Level Quantification of Protein Stability in Living Cells. PNAS 2014, 111, 11335–11340.
- Davis, C.M.; Gruebele, M.; Sukenik, S. How Does Solvation in the Cell Affect Protein Folding and Binding? Curr. Opin. Struct. Biol. 2018, 48, 23–29.
- Ebbinghaus, S.; Dhar, A.; McDonald, J.D.; Gruebele, M. Protein Folding Stability and Dynamics Imaged in a Living Cell. Nat. Methods 2010, 7, 319–323.
- Feng, R.; Gruebele, M.; Davis, C.M. Quantifying Protein Dynamics and Stability in a Living Organism. Nat. Commun. 2019, 10, 1179.
- Guo, M.; Xu, Y.; Gruebele, M. Temperature Dependence of Protein Folding Kinetics in Living Cells. PNAS 2012, 109, 17863–17867.
- Barnes, C.O.; Pielak, G.J. In-Cell Protein NMR and Protein Leakage. Proteins 2011, 79, 347–351.
- Munishkina, L.A.; Ahmad, A.; Fink, A.L.; Uversky, V.N. Guiding Protein Aggregation with Macromolecular Crowding. Biochemistry 2008, 47, 8993–9006.
- Shtilerman, M.D.; Ding, T.T.; Lansbury, P.T.J. Molecular Crowding Accelerates Fibrillization of Alpha-Synuclein: Could an Increase in the Cytoplasmic Protein Concentration Induce Parkinson’s Disease? Biochemistry 2002, 41, 3855–3860.
- Wu, Y.; Teng, N.; Li, S. Effects of Macromolecular Crowding and Osmolyte on Human Tau Fibrillation. Int. J. Biol. Macromol. 2016, 90, 27–36.
- Mittal, S.S. Macromolecular Crowding Decelerates Aggregation of a β-Rich Protein, Bovine Carbonic Anyhdrase: A Case Study. J. Biochem. 2014, 156, 273–282.
- Seeliger, J.; Werkmüller, A.; Winter, R. Macromolecular Crowding as Suppressor of Human IAPP Fibril Formation and Cytotoxicity. PLoS ONE 2013, 8, e69652.
- Ma, Q.; Fan, J.-B.; Zhou, Z.; Zhou, B.-R.; Meng, S.-R.; Hu, J.-Y.; Chen, J.; Liang, Y. The Contrasting Effect of Macromolecular Crowding on Amyloid Fibril Formation. PLoS ONE 2012, 7, e36288.
- Siddiqui, G.A.; Naeem, A. The Contrasting Effect of Macromolecular Crowding and Confinement on Fibril Formation of Globular Protein: Underlying Cause of Proteopathies. J. Mol. Liq. 2021, 322, 114602.
- Ma, B.; Xie, J.; Wei, L.; Li, W. Macromolecular Crowding Modulates the Kinetics and Morphology of Amyloid Self-Assembly by β-Lactoglobulin. Int. J. Biol. Macromol. 2013, 53, 82–87.
- Breydo, L.; Reddy, K.D.; Piai, A.; Felli, I.C.; Pierattelli, R.; Uversky, V.N. The Crowd You’re in with: Effects of Different Types of Crowding Agents on Protein Aggregation. Biochim. Biophys Acta (BBA)-Proteins Proteom. 2014, 1844, 346–357.
- White, D.A.; Buell, A.K.; Knowles, T.P.; Welland, M.E.; Dobson, C.M. Protein Aggregation in Crowded Environments. J. Am. Chem. Soc. 2010, 132, 5170–5175.
- Biswas, S.; Bhadra, A.; Lakhera, S.; Soni, M.; Panuganti, V.; Jain, S.; Roy, I. Molecular Crowding Accelerates Aggregation of α-Synuclein by Altering Its Folding Pathway. Eur. Biophys. J. 2021, 50, 59–67.
- Breydo, L.; Sales, A.E.; Frege, T.; Howell, M.C.; Zaslavsky, B.Y.; Uversky, V.N. Effects of Polymer Hydrophobicity on Protein Structure and Aggregation Kinetics in Crowded Milieu. Biochemistry 2015, 54, 2957–2966.
- Kong, L.-X.Z. Effects of Seeding on Lysozyme Amyloid Fibrillation in the Presence of Epigallocatchin and Polyethylene Glycol. Biochemistry 2017, 82, 266–279.
- Ogston, A.G. The Spaces in a Uniform Random Suspension of Fibres. Trans. Faraday Soc. 1958, 54, 1754–1757.
- Sharp, K.A. Analysis of the Size Dependence of Macromolecular Crowding Shows That Smaller Is Better. Proc. Natl. Acad. Sci. USA 2015, 112, 7990–7995.
- Davis-Searles, P.R.; Saunders, A.J.; Erie, D.A.; Winzor, D.J.; Pielak, G.J. Interpreting the Effects of Small Uncharged Solutes on Protein-Folding Equilibria. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 271–306.
- Knowles, T.P.J.; Waudby, C.A.; Devlin, G.L.; Cohen, S.I.A.; Aguzzi, A.; Vendruscolo, M.; Terentjev, E.M.; Welland, M.E.; Dobson, C.M. An Analytical Solution to the Kinetics of Breakable Filament Assembly. Science 2009, 326, 1533–1537.
- Uversky, V.N.; Li, J.; Fink, A.L. Trimethylamine-N-Oxide-Induced Folding of α-Synuclein. FEBS Lett. 2001, 509, 31–35.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
22 Jul 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




