
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sunday Oyinbo | -- | 5419 | 2022-07-05 09:42:28 | | | |
| 2 | Beatrix Zheng | -2 word(s) | 5417 | 2022-07-06 04:03:40 | | |
Video Upload Options
Thin superconducting films have been a significant part of superconductivity research for more than six decades. They have had a significant impact on the existing consensus on the microscopic and macroscopic nature of the superconducting state. Thin-film superconductors are frequently considered to be Type II superconductors even when they are from Type I materials because of the strong effect of the stray magnetic fields outside the superconductive sample. Thin films can be defined as materials, where one dimension is highly constrained relative to the other two dimensions or a system whose properties are determined by the surface energy. Thin films consist of two main components: the microstructure and the surface morphology. The microstructure refers to the microscopic crystal structure of the thin film. Thin films fabrication has a virtually unlimited ability to synthesise materials with new or improved properties. This means new devices and applications can be realized.
1. Fe-Based Thin-Film Superconductors
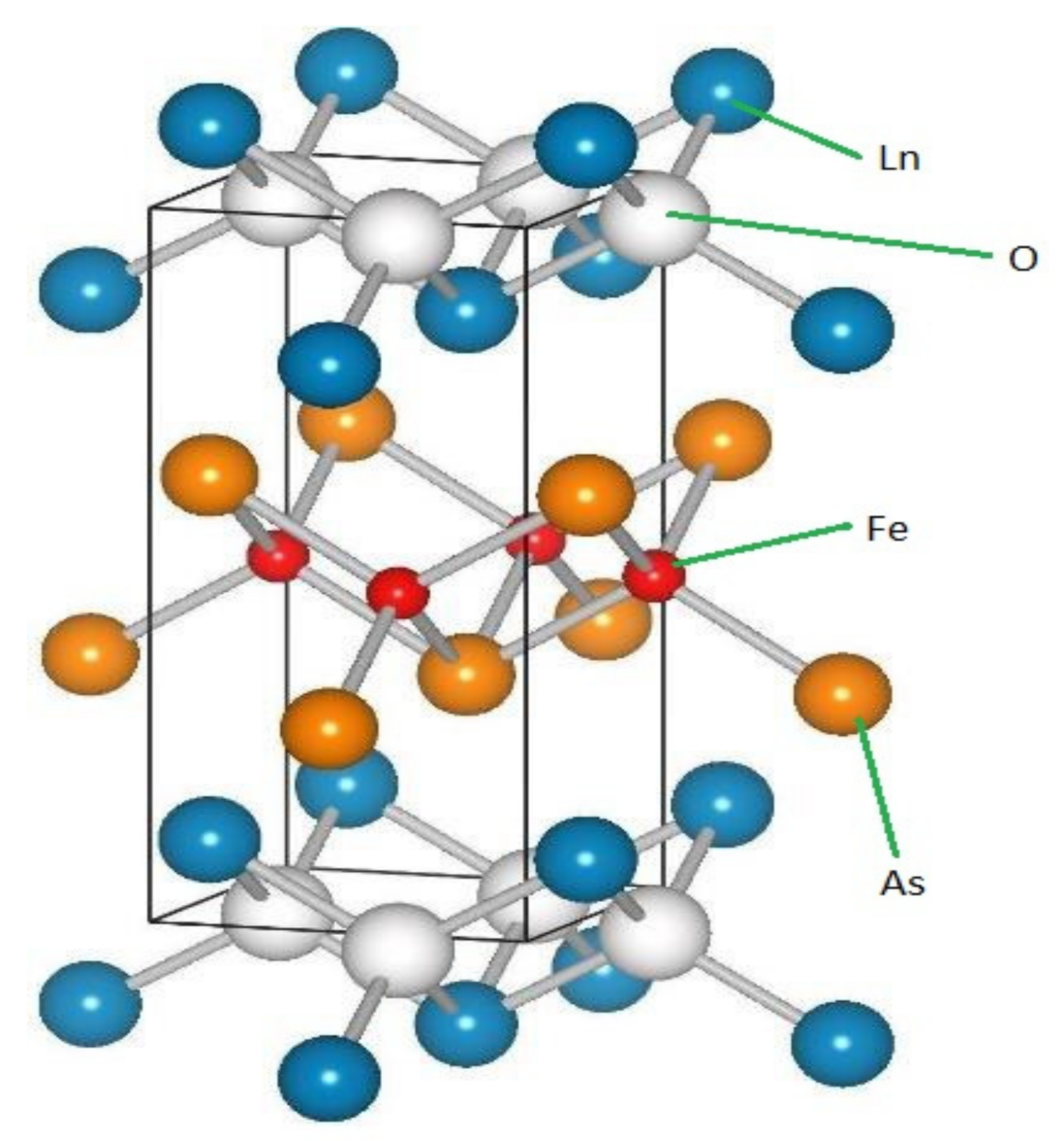
1.1. LnFeAs(O,F) Family
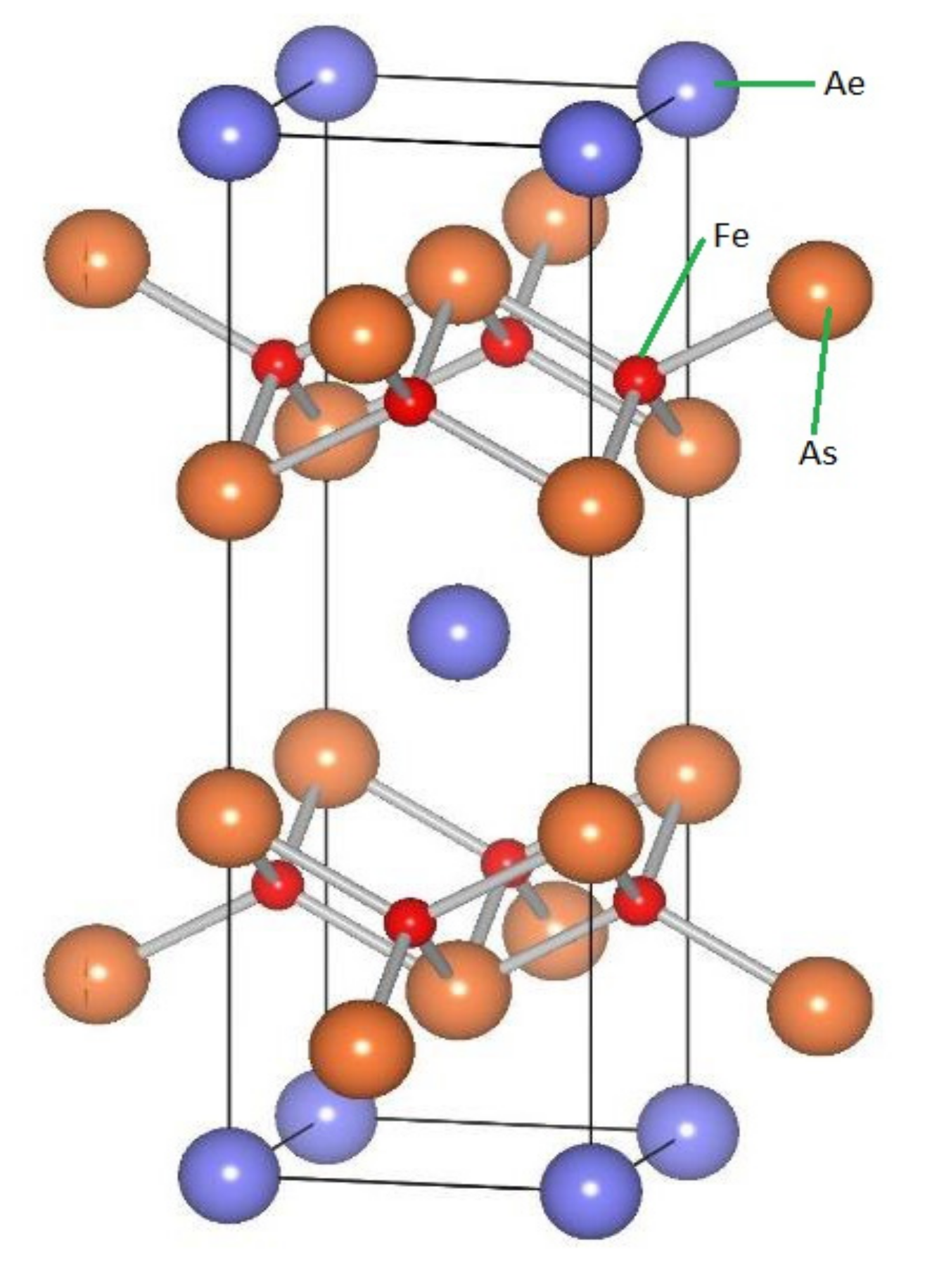
1.2. Doped AeFe2As2 family
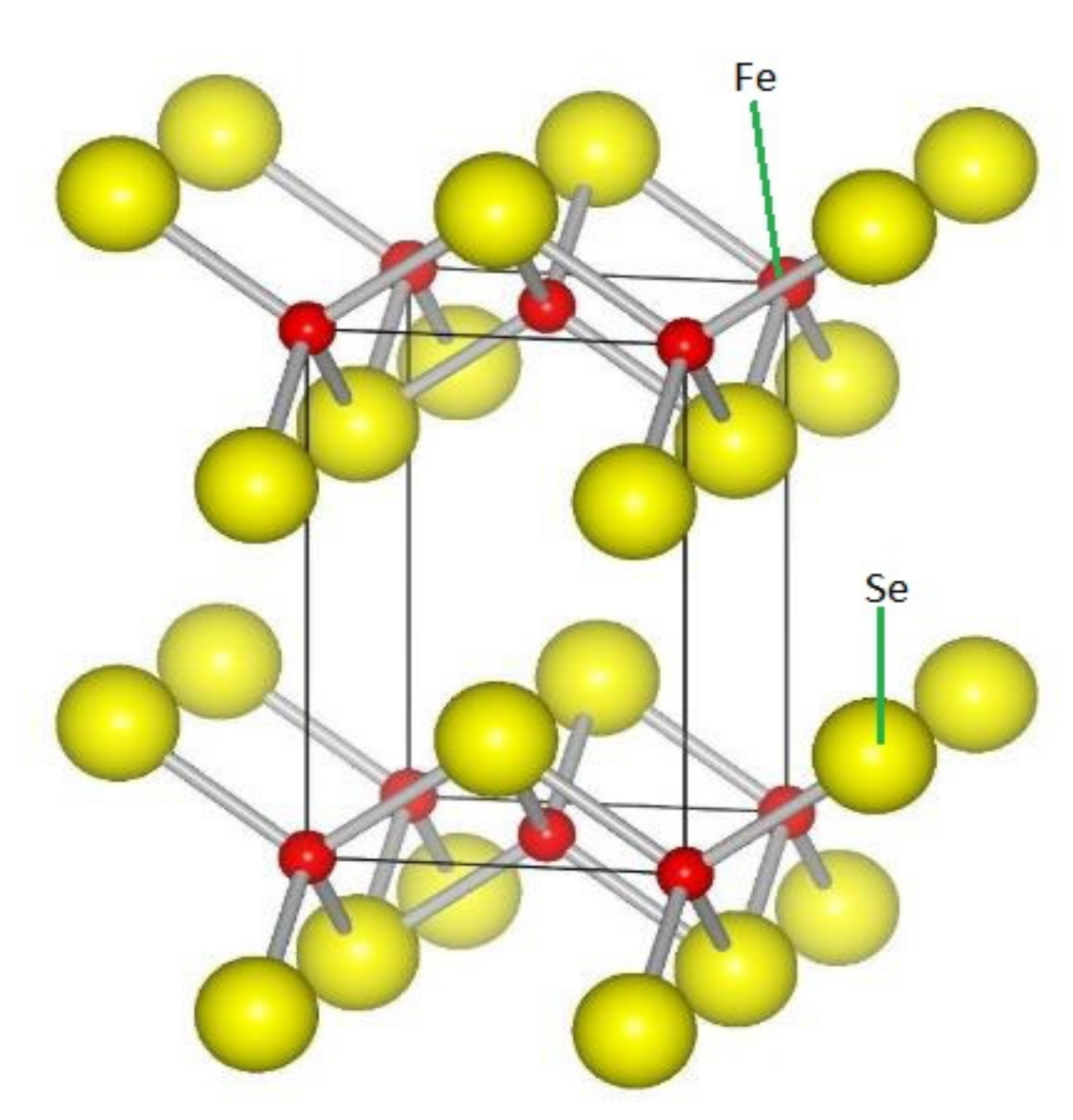
1.3. FeCh and FeSe Mono-Layer Film Family
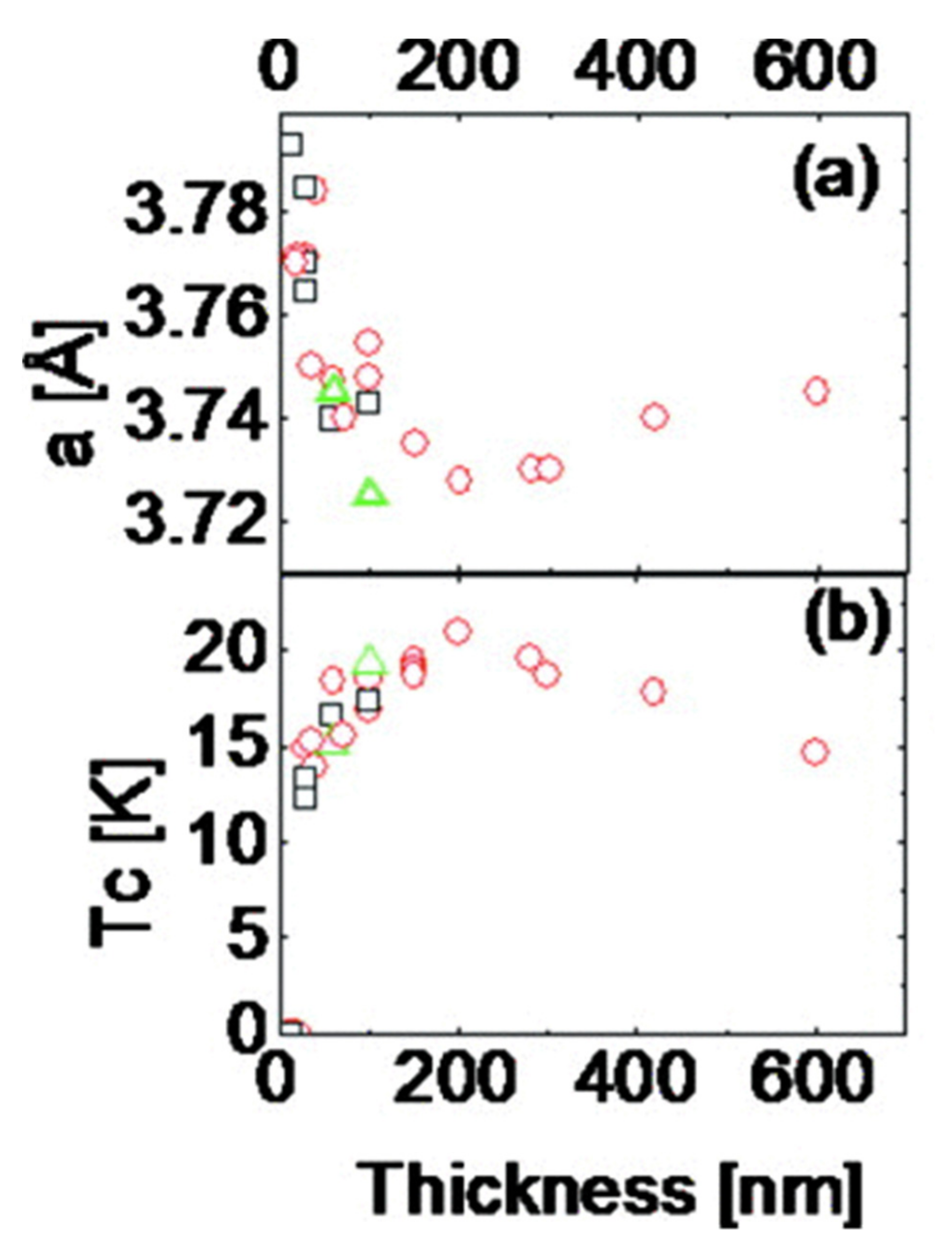
| Materials | Substrate | Method | Transition Temp (K) | References |
|---|---|---|---|---|
| FeSe | r-cut Al2O3 | MBE | Tc(onset) = 13 K | [32] |
| CaF2 | PLD | Tc(onset)= 12.4 K, Tc(end) = 11.9 K | [43] | |
| mono-layer FeSe |
SrTiO3 | MBE | Tc = 42 K | [44] |
| SrTiO3 | Tc = 65 ± 5 K | [45] | ||
| Nb-doped SrTiO3 | Tc = 109 K | [46] | ||
| FeSe1−xTex x = 0.0~1.0 |
MgO | PLD | Tc(onset)~14 K, Tc(end) ~ 12 K at x = 0.5 | [36] |
| FeSe0.5Te0.5 | SrTiO3 | PLD | Tc = 17 K | [47] |
| FeSe0.5Te0.5 | LaAlO3 | PLD | Tc = 21 K | [47] |
| FeSe0.5Te0.5 | CaF2 | PLD | Tc(onset) = 16.3 K, Tc(end) = 15.3 K | [25] |
| FeSe0.8Te0.2 | PLD | Tc = 23 K | [48] | |
| FeSe0.72Te0.18 | PLD | Tc(onset) = 22 K, Tc(end) = 20.5 K | [49] | |
| FeSe0.5Te0.5 | SrF2 | PLD | Tc = 15.7 K | [50] |
| FeSe0.5Te0.5 | BaF2 | PLD | Tc = 12.8 K | |
| FeSe0.5Te0.5 | Fe-buffered MgO | PLD | Tc = 17.7 K | [1][51] |
| FeSe0.5Te0.5 | CeO2-buffered YSZ, RABiTS | PLD | Tc(onset) = 20 K, Tc(end) = 18 K | [52] |
| FeSe0.5Te0.5 | CeO2-buffered SrTiO3 | PLD | Tc(onset) = 18.5 K, Tc(end) = 18 K | [53] |
| FeSe0.5Te0.5 | FeSe1−xTex-buffered MgO | PLD | Tc(onset) ≥ 17 K | [54] |
2. Layered Titanium Compounds
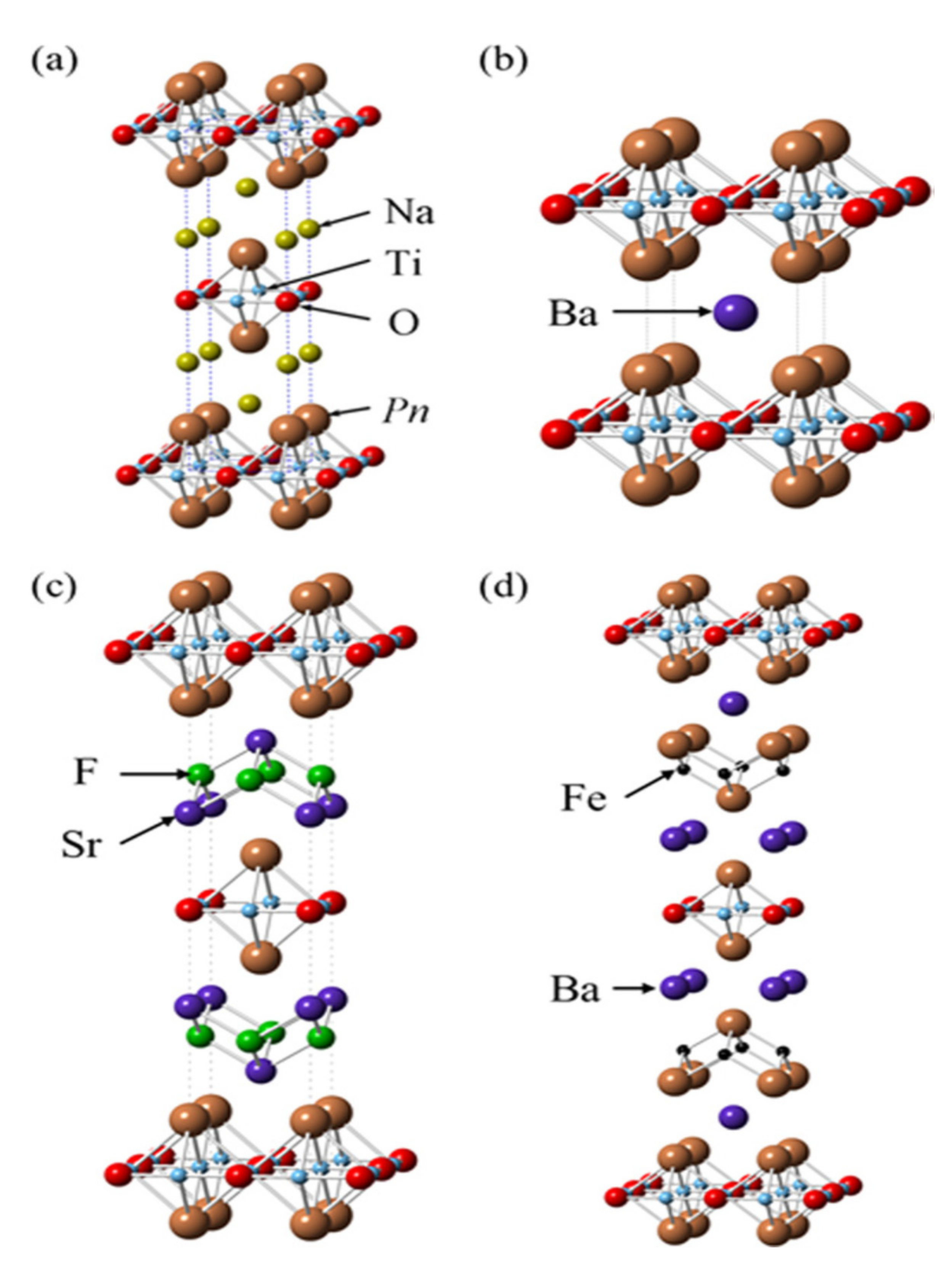
3. Intercalation Compounds with Layered and Cage-like Structures
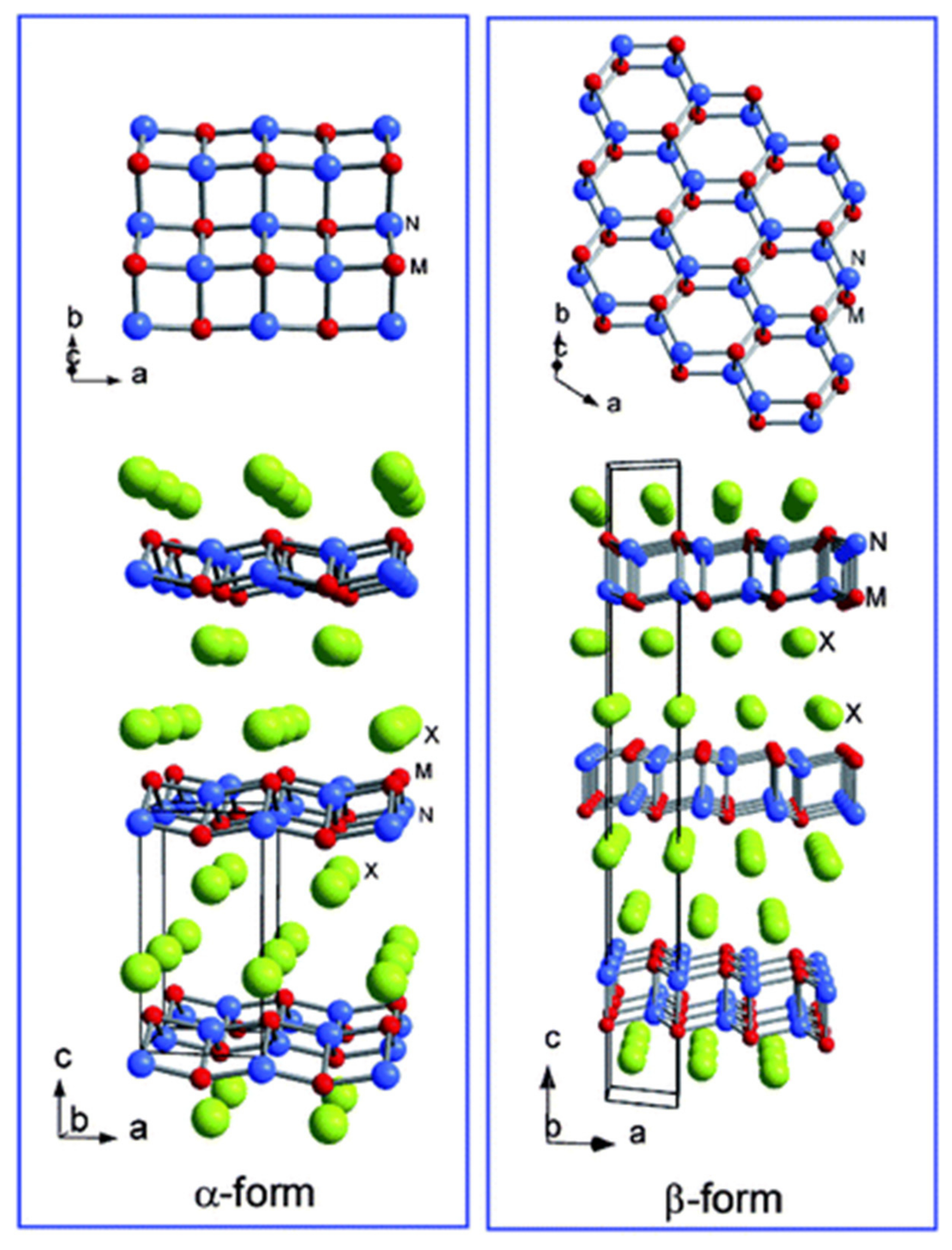
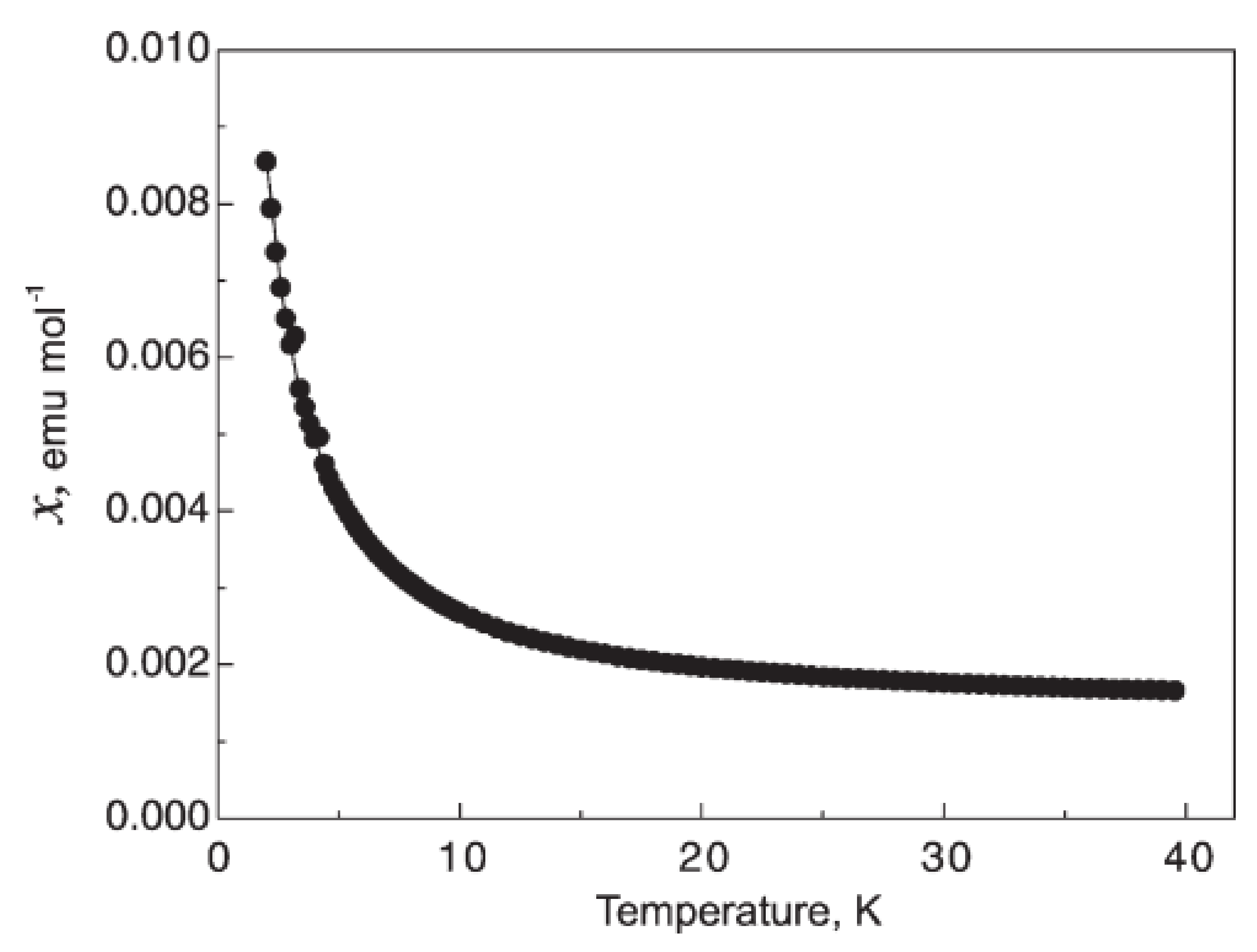
4. Other Superconductors
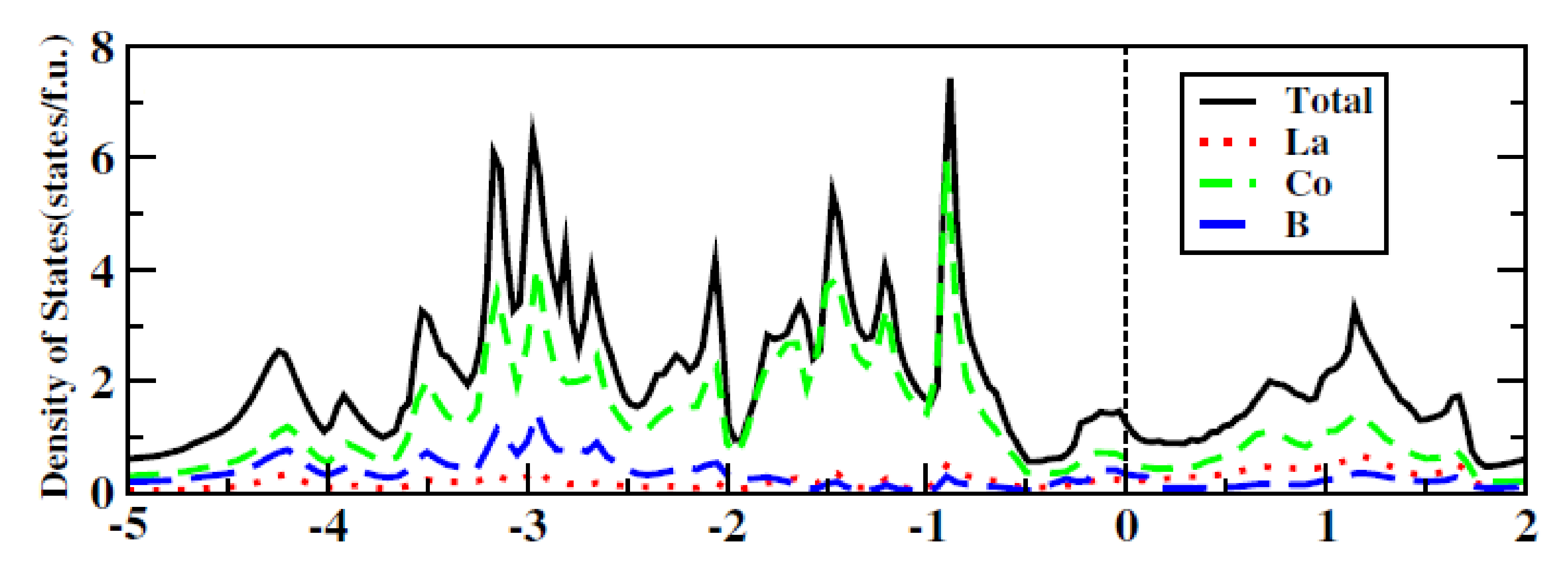
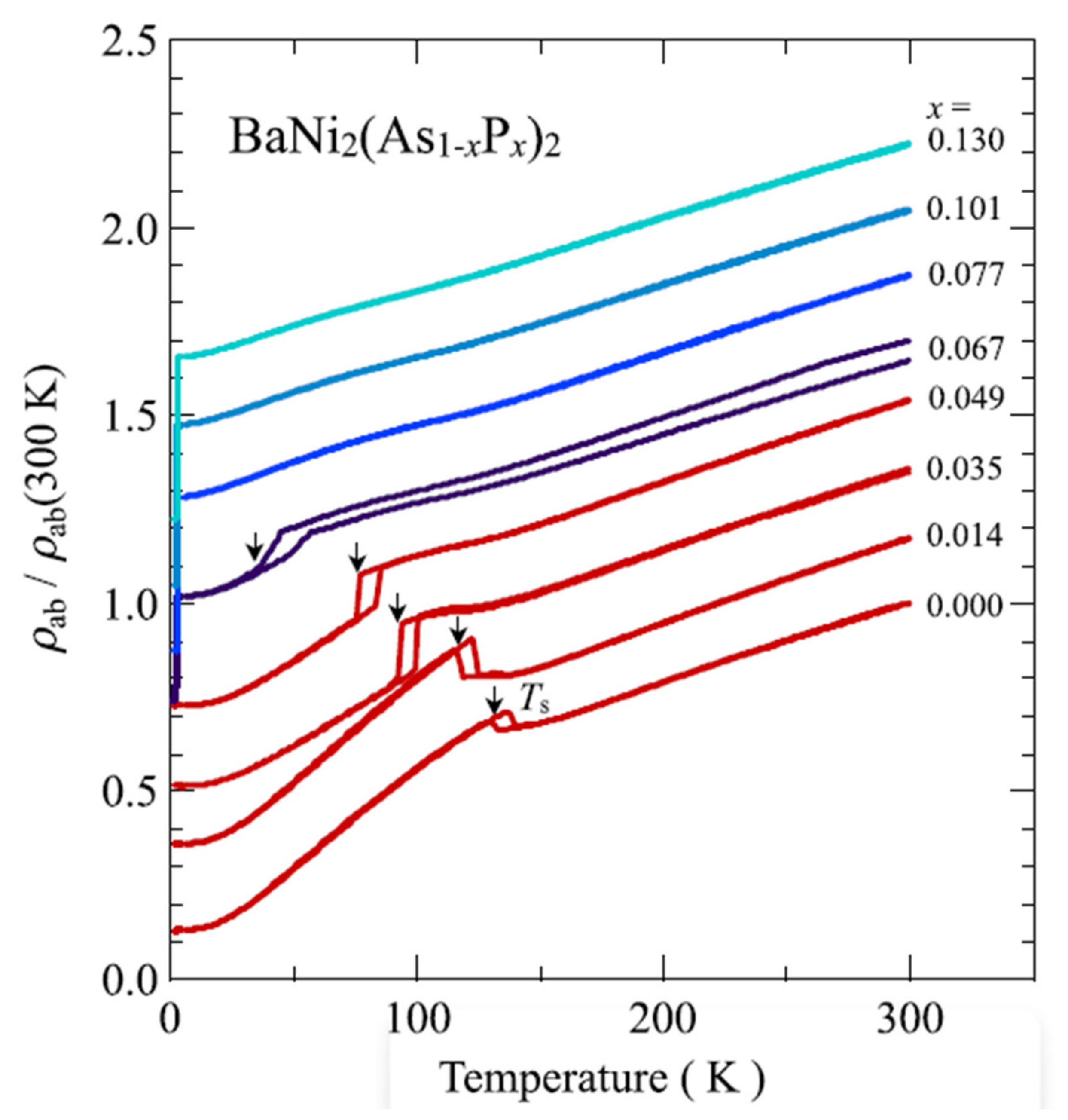
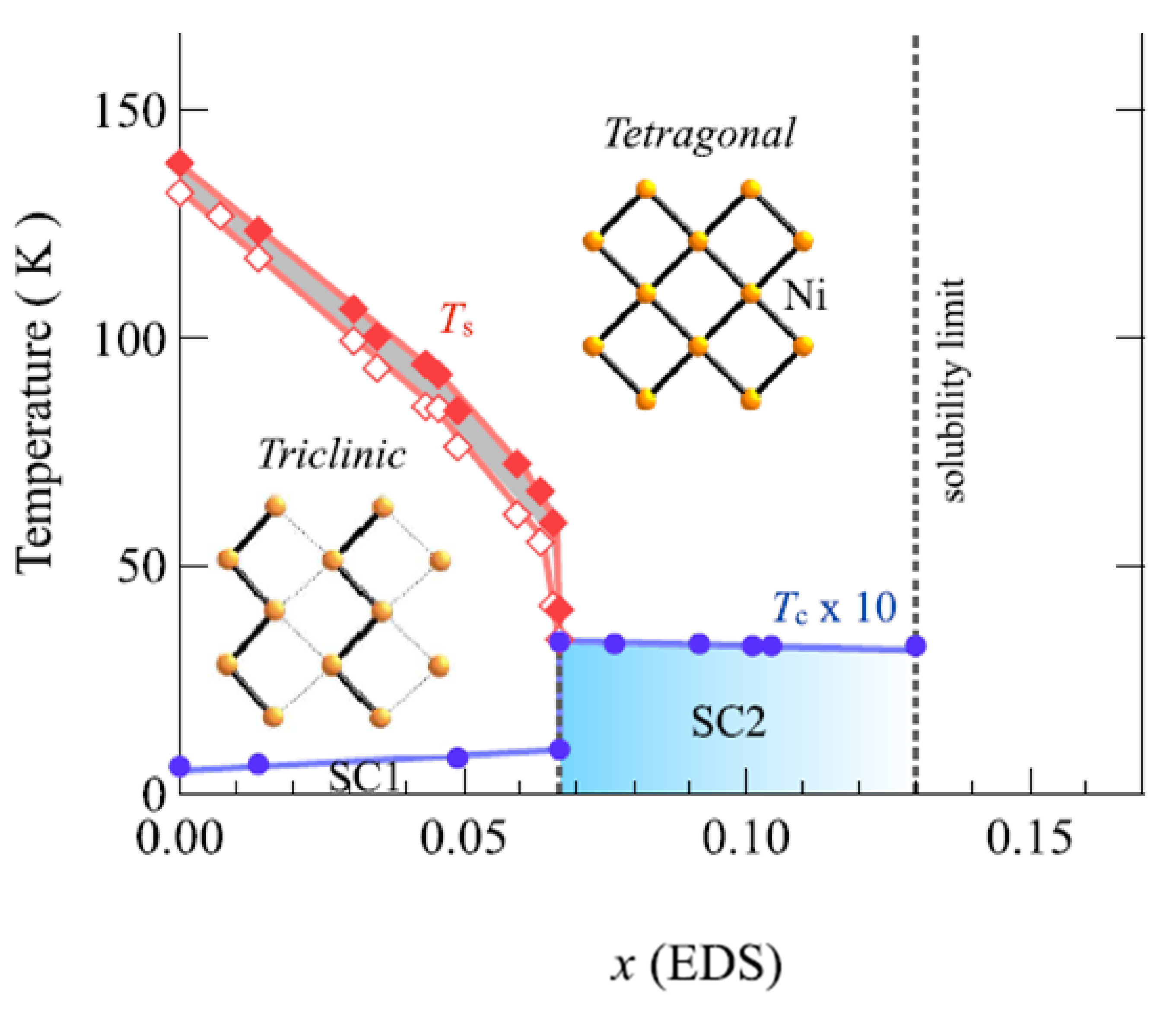
References
- Sakoda, M.; Iida, K.; Naito, M. Recent progress in thin-film growth of Fe-based superconductors: Superior superconductivity achieved by thin films. Supercond. Sci. Technol. 2018, 31, 093001.
- Imai, Y.; Nabeshima, F.; Maeda, A. Comparative Review on Thin Film Growth of Iron-Based Superconductors. Condens. Matter 2017, 2, 25.
- Kamihara, Y.; Watanabe, T.; Hirano, M.; Hosono, H. Iron-Based Layered Superconductor LaFeAs (x = 0.05 − 0.12) with Tc = 26 K. J. Am. Chem. Soc. 2008, 130, 3296–3297.
- Iida, K.; Hänisch, J.; Tarantini, C. Fe-based superconducting thin films on metallic substrates: Growth, characteristics, and relevant properties. Appl. Phys. Rev. 2018, 5, 031304.
- Virtanen, P.; Braggio, A.; Giazotto, F. Superconducting size effect in thin films under electric field: Mean-field self-consistent model. Phys. Rev. B 2019, 100, 224506.
- Antoine, C.Z.; Berry, S.; Bouat, S.; Jacquot, J.-F.; Villégier, J.-C.; Lamura, G.; Gurevich, A. Characterization of superconducting nanometric multilayer samples for superconducting rf applications: First evidence of magnetic screening effect. Phys. Rev. Spéc. Top. Accel. Beams 2010, 13, 121001.
- Hiramatsu, H.; Kamiya, T.; Hirano, M.; Hosono, H. Heteroepitaxial film growth of layered compounds with the ZrCuSiAs-type and ThCr2Si2-type structures: From Cu-based semiconductors to Fe-based superconductors. Phys. C Supercond. 2009, 469, 657–666.
- Hiramatsu, H.; Katase, T.; Kamiya, T.; Hosono, H. Thin Film Growth and Device Fabrication of Iron-Based Superconductors. J. Phys. Soc. Jpn. 2012, 81, 1–25.
- Tanabe, K.; Hosono, H. Frontiers of Research on Iron-Based Superconductors toward Their Application. Jpn. J. Appl. Phys. 2011, 51, 10005.
- Mele, P. Superconducting properties of iron chalcogenide thin films. Sci. Technol. Adv. Mater. 2012, 13, 054301.
- Haindl, S.; Kidszun, M.; Oswald, S.; Hess, C.; Büchner, B.; Kölling, S.; Wilde, L.; Thersleff, T.; Yurchenko, V.V.; Jourdan, M.; et al. Thin film growth of Fe-based superconductors: From fundamental properties to functional devices. A comparative review. Rep. Prog. Phys. 2014, 77, 046502.
- Sadovskii, M.V. High-temperature superconductivity in monolayers FeSe. Uspekhi Fiz. Nauk 2016, 186, 1035–1057.
- Hosono, H.; Yamamoto, A.; Hiramatsu, H.; Ma, Y. Recent advances in iron-based superconductors toward applications. Mater. Today 2018, 21, 278–302.
- Hosono, H.; Tanabe, K.; Takayama-Muromachi, E.; Kageyama, H.; Yamanaka, S.; Kumakura, H.; Nohara, M.; Hiramatsu, H.; Fujitsu, S. Exploration of new superconductors and functional materials, and fabrication of superconducting tapes and wires of iron pnictides. Sci. Technol. Adv. Mater. 2015, 16, 033503.
- Watanabe, T.; Yanagi, H.; Kamihara, Y.; Kamiya, T.; Hirano, M.; Hosono, H. Nickel-based layered superconductor, LaNiOAs. J. Solid State Chem. 2008, 181, 2117–2120.
- Hsu, F.-C.; Luo, J.-Y.; Yeh, K.-W.; Chen, T.-K.; Huang, T.-W.; Wu, P.M.; Lee, Y.-C.; Huang, Y.-L.; Chu, Y.-Y.; Yan, D.-C.; et al. Superconductivity in the PbO-type structure α-FeSe. Proc. Natl. Acad. Sci. USA 2008, 105, 14262–14264.
- Kamihara, Y.; Hiramatsu, H.; Hirano, M.; Kawamura, R.; Yanagi, H.; Kamiya, T.; Hosono, H. Iron-Based Layered Superconductor: LaOFeP. J. Am. Chem. Soc. 2006, 128, 10012–10013.
- Watanabe, T.; Yanagi, H.; Kamiya, T.; Kamihara, Y.; Hiramatsu, H.; Hirano, M.; Hosono, H. Nickel-Based Oxyphosphide Superconductor with a Layered Crystal Structure, LaNiOP. Inorg. Chem. 2007, 46, 7719–7721.
- Ren, Z.-A.; Che, G.-C.; Dong, X.-L.; Yang, J.; Lu, W.; Yi, W.; Shen, X.-L.; Li, Z.-C.; Sun, L.-L.; Zhou, F.; et al. Superconductivity and phase diagram in iron-based arsenic-oxides ReFeAsO 1−δ (Re = rare-earth metal) without fluorine doping. Eur. Lett. 2008, 83, 17002.
- Kawaguchi, T.; Uemura, H.; Ohno, T.; Watanabe, R.; Tabuchi, M.; Ujihara, T.; Takenaka, K.; Takeda, Y.; Ikuta, H. Epitaxial Growth of NdFeAsO Thin Films by Molecular Beam Epitaxy. Appl. Phys. Express 2009, 2, 093002.
- Kawaguchi, T.; Uemura, H.; Ohno, T.; Tabuchi, M.; Ujihara, T.; Takenaka, K.; Takeda, Y.; Ikuta, H. In situ growth of superconducting NdFeAs(O,F) thin films by molecular beam epitaxy. Appl. Phys. Lett. 2010, 97, 042509.
- Backen, E.; Haindl, S.; Niemeier, T.; Hühne, R.; Freudenberg, T.; Werner, J.; Behr, G.; Schultz, L.; Holzapfel, B. Growth and anisotropy of La(O, F)FeAs thin films deposited by pulsed laser deposition. Supercond. Sci. Technol. 2008, 21, 122001.
- Kidszun, M.; Haindl, S.; Reich, E.; Hänisch, J.; Iida, K.; Schultz, L.; Holzapfel, B. Epitaxial LaFeAsO1−xFx thin films grown by pulsed laser deposition. Supercond. Sci. Technol. 2010, 23, 022002.
- Ueda, S.; Takeda, S.; Takano, S.; Yamamoto, A.; Naito, M. High-Tc and high-Jc SmFeAs(O,F) films on fluoride substrates grown by molecular beam epitaxy. Appl. Phys. Lett. 2011, 99, 232505.
- Tsukada, I.; Hanawa, M.; Akiike, T.; Nabeshima, F.; Imai, Y.; Ichinose, A.; Komiya, S.; Hikage, T.; Kawaguchi, T.; Ikuta, H.; et al. Epitaxial Growth of FeSe0.5Te0.5Thin Films on CaF2Substrates with High Critical Current Density. Appl. Phys. Express 2011, 4, 053101.
- Haindl, S.; Hanzawa, K.; Sato, H.; Hiramatsu, H.; Hosono, H. In-situ growth of superconducting SmO1−xFxFeAs thin films by pulsed laser deposition. Sci. Rep. 2016, 6, 35797.
- Kamihara, Y.; Nomura, T.; Hirano, M.; Kim, J.E.; Kato, K.; Takata, M.; Kobayashi, Y.; Kitao, S.; Higashitaniguchi, S.; Yoda, Y.; et al. Electronic and magnetic phase diagram of superconductors, SmFeAsO1−xFx. New J. Phys. 2010, 12, 1–14.
- Rotter, M.; Pangerl, M.; Tegel, M.; Johrendt, D. Superconductivity and Crystal Structures of (Ba1−xKx)Fe2As2(x = 0–1). Angew. Chem. Int. Ed. 2008, 47, 7949–7952.
- Rotter, M.; Tegel, M.; Johrendt, D. Superconductivity at 38 K in the Iron Arsenide(Ba1−xKx)Fe2As2. Phys. Rev. Lett. 2008, 101, 107006.
- Lee, N.H.; Jung, S.-G.; Kim, D.H.; Kang, W. Potassium-doped BaFe2As2 superconducting thin films with a transition temperature of 40 K. Appl. Phys. Lett. 2010, 96, 202505.
- Lee, N.H.; Jung, S.-G.; Ranot, M.; Kang, W. Fabrication details of Ba1−xKxFe2As2 films by pulsed laser deposition technique. Prog. Supercond. Cryog. 2014, 16, 4–6.
- Agatsuma, S.; Yamagishi, T.; Takeda, S.; Naito, M. MBE growth of FeSe and Sr1−xKxFe2As2. Phys. C 2010, 470, 1468–1472.
- Takeda, S.; Ueda, S.; Yamagishi, T.; Agatsuma, S.; Takano, S.; Mitsuda, A.; Naito, M. Molecular Beam Epitaxy Growth of Superconducting Sr1−xKxFe2As2 and Ba1−xKxFe2As2. Appl. Phys. Express 2010, 3, 093101.
- Ueda, S.; Yamagishi, T.; Takeda, S.; Agatsuma, S.; Takano, S.; Mitsuda, A.; Naito, M. MBE growth of Fe-based superconducting films. Phys. C Supercond. 2011, 471, 1167–1173.
- Yamagishi, T.; Ueda, S.; Takeda, S.; Takano, S.; Mitsuda, A.; Naito, M. A study of the doping dependence of Tc in Ba1−xKxFe2As2 and Sr1−xKxFe2As2 films grown by molecular beam epitaxy. Phys. C Supercond. 2011, 471, 1177–1180.
- Wu, M.K.; Hsu, F.C.; Yeh, K.W.; Huang, T.W.; Luo, J.Y.; Wang, M.J.; Chang, H.H.; Chen, T.K.; Rao, S.M.; Mok, B.H.; et al. The development of the superconducting PbO-type β-FeSe and related compounds. Phys. C 2009, 469, 340–349.
- Yeh, K.-W.; Huang, T.-W.; Huang, Y.-L.; Chen, T.-K.; Hsu, F.-C.; Wu, P.M.; Lee, Y.-C.; Chu, Y.-Y.; Chen, C.-L.; Luo, J.-Y.; et al. Tellurium substitution effect on superconductivity of the α-phase iron selenide. Eur. Lett. 2008, 84, 104502.
- Mizuguchi, Y.; Tomioka, F.; Tsuda, S.; Yamaguchi, T.; Takano, Y. Superconductivity at 27K in tetragonal FeSe under high pressure. Appl. Phys. Lett. 2008, 93, 152505.
- Medvedev, S.; McQueen, T.M.; Troyan, I.A.; Palasyuk, T.; Eremets, M.I.; Cava, R.J.; Naghavi, S.; Casper, F.; Ksenofontov, V.; Wortmann, G.; et al. Electronic and magnetic phase diagram of β-Fe1.01Se with superconductivity at 36.7 K under pressure. Nat. Mater. 2009, 8, 630–633.
- Bellingeri, E.; Kawale, S.; Pallecchi, I.; Gerbi, A.; Buzio, R.; Braccini, V.; Palenzona, A.; Putti, M.; Adamo, M.; Sarnelli, E.; et al. Strong vortex pinning in FeSe0.5Te0.5 epitaxial thin film. Appl. Phys. Lett. 2012, 100, 082601.
- Hanzawa, K.; Sato, H.; Hiramatsu, H.; Kamiya, T.; Hosono, H. Electric field-induced superconducting transition of insulating FeSe thin film at 35 K. Proc. Natl. Acad. Sci. USA 2016, 113, 3986–3990.
- Lei, B.; Cui, J.H.; Xiang, Z.J.; Shang, C.; Wang, N.Z.; Ye, G.J.; Luo, X.G.; Wu, T.; Sun, Z.; Chen, X.H. Evolution of High-Temperature Superconductivity from a Low-TcPhase Tuned by Carrier Concentration in FeSe Thin Flakes. Phys. Rev. Lett. 2016, 116, 077002.
- Nabeshima, F.; Imai, Y.; Hanawa, M.; Tsukada, I.; Maeda, A. Enhancement of the superconducting transition temperature in FeSe epitaxial thin films by anisotropic compression. Appl. Phys. Lett. 2013, 103, 172602.
- Wang, Q.-Y.; Li, Z.; Zhang, W.-H.; Zhang, Z.-C.; Zhang, J.-S.; Li, W.; Ding, H.; Ou, Y.-B.; Deng, P.; Chang, K.; et al. Interface-Induced High-Temperature Superconductivity in Single Unit-Cell FeSe Films on SrTiO3. Chin. Phys. Lett. 2012, 29, 037402.
- He, S.; He, J.; Zhang, W.; Zhao, L.; Liu, D.; Liu, X.; Mou, D.; Ou, Y.-B.; Wang, Q.-Y.; Li, Z.; et al. Phase diagram and electronic indication of high-temperature superconductivity at 65 K in single-layer FeSe films. Nat. Mater. 2013, 12, 605–610.
- Ge, J.-F.; Liu, Z.-L.; Liu, C.; Gao, C.-L.; Qian, D.; Xue, Q.-K.; Liu, Y.; Jia, J.-F. Superconductivity above 100 K in single-layer FeSe films on doped SrTiO3. Nat. Mater. 2015, 14, 285–289.
- Putti, M.; Pallecchi, I.; Bellingeri, E.; Cimberle, M.R.; Tropeano, M.; Ferdeghini, C.; Palenzona, A.; Tarantini, C.; Yamamoto, A.; Jiang, J.; et al. New Fe-based superconductors: Properties relevant for applications. Supercond. Sci. Technol. 2010, 23, 034003.
- Imai, Y.; Sawada, Y.; Nabeshima, F.; Maeda, A. Suppression of phase separation and giant enhancement of superconducting transition temperature in FeSe1−x Tex thin films. Proc. Natl. Acad. Sci. USA 2015, 112, 1937–1940.
- Seo, S.; Kang, J.-H.; Oh, M.J.; Jeong, I.-S.; Jiang, J.; Gu, G.; Lee, J.-W.; Lee, J.; Noh, H.; Liu, M.; et al. Origin of the emergence of higher Tc than bulk in iron chalcogenide thin films. Sci. Rep. 2017, 7, 1–8.
- Hanawa, M.; Ichinose, A.; Komiya, S.; Tsukada, I.; Imai, Y.; Maeda, A. Empirical selection rule of substrate materials for iron chalcogenide superconducting thin films. Japan. J. Appl. Phys. 2012, 51, 010104.
- Iida, K.; Hänisch, J.; Schulze, M.; Aswartham, S.; Wurmehl, S.; Büchner, B.; Schultz, L.; Holzapfel, B. Generic Fe buffer layers for Fe-based superconductors: Epitaxial FeSe1−xTex thin films. Appl. Phys. Lett. 2011, 99, 202503.
- Si, W.; Han, S.J.; Shi, X.; Ehrlich, S.N.; Jaroszynski, J.; Goyal, A.; Li, Q. High current superconductivity in FeSe0.5Te0.5-coated conductors at 30 tesla. Nat. Commun. 2013, 4, 1347.
- Ozaki, T.; Wu, L.; Zhang, C.; Jaroszynski, J.; Si, W.; Zhou, J.; Zhu, Y.; Li, Q. A route for a strong increase of critical current in nanostrained iron-based superconductors. Nat. Commun. 2016, 7, 13036.
- Molatta, S.; Haindl, S.; Trommler, S.; Schulze, M.; Wurmehl, S.; Hühne, R. Interface control by homoepitaxial growth in pulsed laser deposited iron chalcogenide thin films. Sci. Rep. 2015, 5, 16334.
- Ozawa, T.C.; Kauzlarich, S.M.; Bieringer, M.; Greedan, J.E. Possible Charge-Density-Wave/Spin-Density-Wave in the Layered Pnictide−Oxides: Na2Ti2Pn2O (Pn = As, Sb). Chem. Mater. 2001, 13, 1804–1810.
- Liu, R.H.; Tan, D.; Song, Y.A.; Li, Q.J.; Yan, Y.J.; Ying, J.J.; Xie, Y.L.; Wang, X.F.; Chen, X.H. Physical properties of the layered pnictide oxidesNa2Ti2P2O (P = As,Sb). Phys. Rev. B 2009, 80, 144516.
- Lorenz, B.; Guloy, A.M.; Chu, P.C.W. Superconductivity in titanium-based pnictide oxide compounds. Int. J. Mod. Phys. B 2014, 28, 1–25.
- Yajima, T.; Nakano, K.; Takeiri, F.; Ono, T.; Hosokoshi, Y.; Matsushita, Y.; Hester, J.; Kobayashi, Y.; Kageyama, H. Superconductivity in BaTi2Sb2O with ad1Square Lattice. J. Phys. Soc. Jpn. 2012, 81, 1–4.
- Doan, P.; Gooch, M.; Tang, Z.; Lorenz, B.; Möller, A.; Tapp, J.; Chu, P.C.W.; Guloy, A.M. Ba1−xNaxTi2Sb2O (0.0 ≤ x ≤ 0.33): A Layered Titanium-Based Pnictide Oxide Superconductor. J. Am. Chem. Soc. 2012, 134, 16520–16523.
- Yajima, T. Titanium Pnictide Oxide Superconductors. Condens. Matter 2017, 2, 4.
- Adam, A.; Schuster, H.-U. Darstellung und Kristallstruktur der Pnictidoxide Na2Ti2As2O und Na2Ti2Sb2O. ZAAC 1990, 584, 150–158.
- Wang, X.; Yan, Y.J.; Ying, J.J.; Li, Q.J.; Zhang, M.; Xu, N.; Chen, X.H. Structure and physical properties for a new layered pnictide-oxide: BaTi2As2O. J. Phys. Condens. Matter 2010, 22, 075702.
- Yajima, T.; Nakano, K.; Takeiri, F.; Hester, J.; Yamamoto, T.; Kobayashi, Y.; Tsuji, N.; Kim, J.; Fujiwara, A.; Kageyama, H. Synthesis and physical properties of the new oxybismuthides BaTi2Bi2O and (SrF)2Ti2Bi2O with a d1 square net. J. Phys. Soc. Jpn. 2012, 82, 013703.
- Liu, R.H.; Song, Y.A.; Li, Q.J.; Ying, J.J.; Yan, Y.J.; He, Y.; Chen, X. Structure and Physical Properties of the Layered Pnictide-Oxides: (SrF)2Ti2Pn2O (Pn= As, Sb) and (SmO)2Ti2Sb2O. Chem. Mater. 2010, 22, 1503–1508.
- Singh, D.J. Electronic structure, disconnected Fermi surfaces and antiferromagnetism in the layered pnictide superconductor NaxBa1−xTi2Sb2O. New J. Phys. 2012, 14, 123003.
- Suetin, D.V.; Ivanovskii, A.L. Electronic properties and fermi surface for new Fe-free layered pnictide-oxide superconductor BaTi2Bi2O from first principles. JETP Lett. 2013, 97, 220–225.
- Bussmann-Holder, A.; Keller, H. High-temperature superconductors: Underlying physics and applications. Z. Für Nat. B 2020, 75, 3–14.
- Müller, K.A.; Kool, T.W. Superconductivity. Prop. Perovskites Other Oxides 2010, 193, 545–562.
- Mine, T.; Yanagi, H.; Kamiya, T.; Kamihara, Y.; Hirano, M.; Hosono, H. Nickel-based phosphide superconductor with infinite-layer structure, BaNi2P2. Solid State Commun. 2008, 147, 111–113.
- Laloë, J.-B.; Kim, T.H.; Moodera, J.S. Molecular-Beam Epitaxially Grown MgB2Thin Films and Superconducting Tunnel Junctions. Adv. Condens. Matter Phys. 2011, 2011, 989732.
- Askerzade, I. The upper critical field of thin films of two-band superconductors: An application to MgB2. Mod. Phys. Lett. B 2004, 18, 1525–1531.
- Yamanaka, S.; Enishi, E.; Fukuoka, H.; Yasukawa, M. High-Pressure Synthesis of a New Silicon Clathrate Superconductor, Ba8Si46. Inorg. Chem. 2000, 39, 56–58.
- Yamanaka, S. Intercalation and superconductivity in ternary layer structured metal nitride halides (MNX: M = Ti, Zr, Hf; X = Cl, Br, I). J. Mater. Chem. 2010, 20, 2922–2933.
- Yamanaka, S.; Okumura, H.; Zhu, L. Alkali metal intercalation in layer structured α-HfNBr☆. J. Phys. Chem. Solids 2004, 65, 565–569.
- Kuroki, K. Spin-fluctuation-mediatedd+id′pairing mechanism in dopedβ-MNCl(M = Hf,Zr)superconductors. Phys. Rev. B 2010, 81, 1–7.
- Bill, A.; Morawitz, H.; Kresin, V.Z. Dynamical screening and superconducting state in intercalated layered metallochloronitrides. Phys. Rev. B 2002, 66, 100501.
- Bill, A.; Morawitz, H.; Kresin, V.Z. Electronic collective modes and superconductivity in layered conductors. Phys. Rev. B 2003, 68, 144519.
- Yamanaka, S.; Hotehama, K.-I.; Kawaji, H. Superconductivity at 25.5 K in electron-doped layered hafnium nitride. Nature 1998, 392, 580–582.
- Yamanaka, S.; Kawaji, H.; Hotehama, K.-I.; Ohashi, M. A new layer-structured nitride superconductor. Lithium-intercalatedβ-zirconium nitride chloride, LixZrNCl. Adv. Mater. 1996, 8, 771–774.
- Yamanaka, S.; Izumi, S.; Maekawa, S.; Umemoto, K. Phase diagram of the La–Si binary system under high pressure and the structures of superconducting LaSi5 and LaSi10. J. Solid State Chem. 2009, 182, 1991–2003.
- Yamanaka, S.; Maekawa, S. Structural Evolution of the Binary System Ba-Si under High-pressure and High-temperature Conditions. Z. Für Nat. B 2006, 61, 1493–1499.
- Kurakevych, O.O.; Strobel, T.A.; Kim, D.Y.; Muramatsu, T.; Struzhkin, V.V. Na-Si Clathrates Are High-Pressure Phases: A Melt-Based Route to Control Stoichiometry and Properties. Cryst. Growth Des. 2013, 13, 303–307.
- Zhang, S.; Tanaka, M.; Watanabe, E.; Zhu, H.; Inumaru, K.; Yamanaka, S. Superconductivity of alkali metal intercalated TiNBr with α-type nitride layers. Supercond. Sci. Technol. 2013, 26, 122001.
- Ronning, F.; Bauer, E.D.; Park, T.; Baek, S.-H.; Sakai, H.; Thompson, J.D. Superconductivity and the effects of pressure and structure in single-crystallineSrNi2P2. Phys. Rev. B 2009, 79, 134507.
- Hirai, D.; Takayama, T.; Higashinaka, R.; Aruga-Katori, H.; Takagi, H. Superconductivity in Layered Pnictides BaRh2P2 and BaIr2P2. J. Phys. Soc. Jpn. 2009, 78, 1–4.
- Jeitschko, W.; Glaum, R.; Boonk, L. Superconducting LaRu2P2 and other alkaline earth and rare earth metal ruthenium and osmium phosphides and arsenides with ThCr2Si2 structure. J. Solid State Chem. 1987, 69, 93–100.
- Han, J.-T.; Zhou, J.-S.; Cheng, J.-G.; Goodenough, J.B. A New Pnictide Superconductor without Iron. J. Am. Chem. Soc. 2009, 132, 908–909.
- Bauer, E.; Ronning, F.; Scott, B.; Thompson, J.D. Superconductivity inSrNi2As2single crystals. Phys. Rev. B 2008, 78, 172504.
- Ronning, F.; Kurita, N.; Bauer, E.; Scott, B.; Park, T.; Klimczuk, T.; Movshovich, R.; Thompson, J.D. The first order phase transition and superconductivity in BaNi2As2single crystals. J. Phys. Condens. Matter 2008, 20, 342203.
- Imre, A.; Hellmann, A.; Wenski, G.; Graf, J.; Johrendt, D.; Mewis, A. Inkommensurabel modulierte Kristallstrukturen und Phasenumwandlungen–Die Verbindungen SrPt2As2 und EuPt2As2. Z. Für Anorg. Und Allg. Chem. 2007, 633, 2037–2045.
- Tsutsumi, K.; Takayanagi, S.; Ishikawa, M.; Hirano, T. Superconductivity of Intermetallic Compound CoSi2. J. Phys. Soc. Jpn. 1995, 64, 2237.
- Sefat, A.; McGuire, M.; Jin, R.; Sales, B.C.; Mandrus, D.; Ronning, F.; Bauer, E.; Mozharivskyj, Y. Structure and anisotropic properties ofBaFe2-xNixAs2(x = 0, 1, and 2) single crystals. Phys. Rev. B 2009, 79, 094508.
- Kurita, N.; Ronning, F.; Tokiwa, Y.; Bauer, E.D.; Subedi, A.; Singh, D.J.; Thompson, J.D.; Movshovich, R. Low-Temperature Magnetothermal Transport Investigation of a Ni-Based SuperconductorBaNi2As2: Evidence for Fully Gapped Superconductivity. Phys. Rev. Lett. 2009, 102, 147004.
- Subedi, A.; Singh, D.J. Density functional study ofBaNi2As2: Electronic structure, phonons, and electron-phonon superconductivity. Phys. Rev. B 2008, 78, 132511.
- Shein, I.R.; Ivanovskii, A.L. Electronic and structural properties of low-temperature superconductors and ternary pnictides ANi2Pn2 (A = Sr, BaandPn = P,As). Phys. Rev. B 2009, 79, 054510.
- Huebener, R. Conductors, Semiconductors and Superconductors: An Introduction to Solid State Physics, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016.
- Kudo, K.; Takasuga, M.; Okamoto, Y.; Hiroi, Z.; Nohara, M. Giant Phonon Softening and Enhancement of Superconductivity by Phosphorus Doping ofBaNi2As2. Phys. Rev. Lett. 2012, 109, 097002.
- Toriyama, T.; Kobori, M.; Konishi, T.; Ohta, Y.; Sugimoto, K.; Kim, J.; Fujiwara, A.; Pyon, S.; Kudo, K.; Nohara, M. Switching of Conducting Planes by Partial Dimer Formation in IrTe2. J. Phys. Soc. Jpn. 2014, 83, 33701.
- Pyon, S.; Kudo, K.; Nohara, M. Emergence of superconductivity near the structural phase boundary in Pt-doped IrTe2 single crystals. Physica C. 2013, 494, 80–84.
- Joseph, B.; Bendele, M.; Simonelli, L.; Maugeri, L.; Pyon, S.; Kudo, K. Local structural displacements across the structural phase transition in IrTe2: Order-disorder of dimers and role of Ir-Te correlations. Phys Rev B. 2013, 88, 3–6.
- Jobic, S.; Evain, M.; Brec, R.; Deniard, P.; Jouanneaux, A.; Rouxel, J. Crystal structure of polymeric pyrite type Ir2Te2. J. Solid State Chem. 1991, 95, 319–326.
- Léger, J.; Pereira, A.; Haines, J.; Jobic, S.; Brec, R. Phase transformations of polymeric CdI2-type IrTe2 under high pressure. J. Phys. Chem. Solids 2000, 61, 27–34.
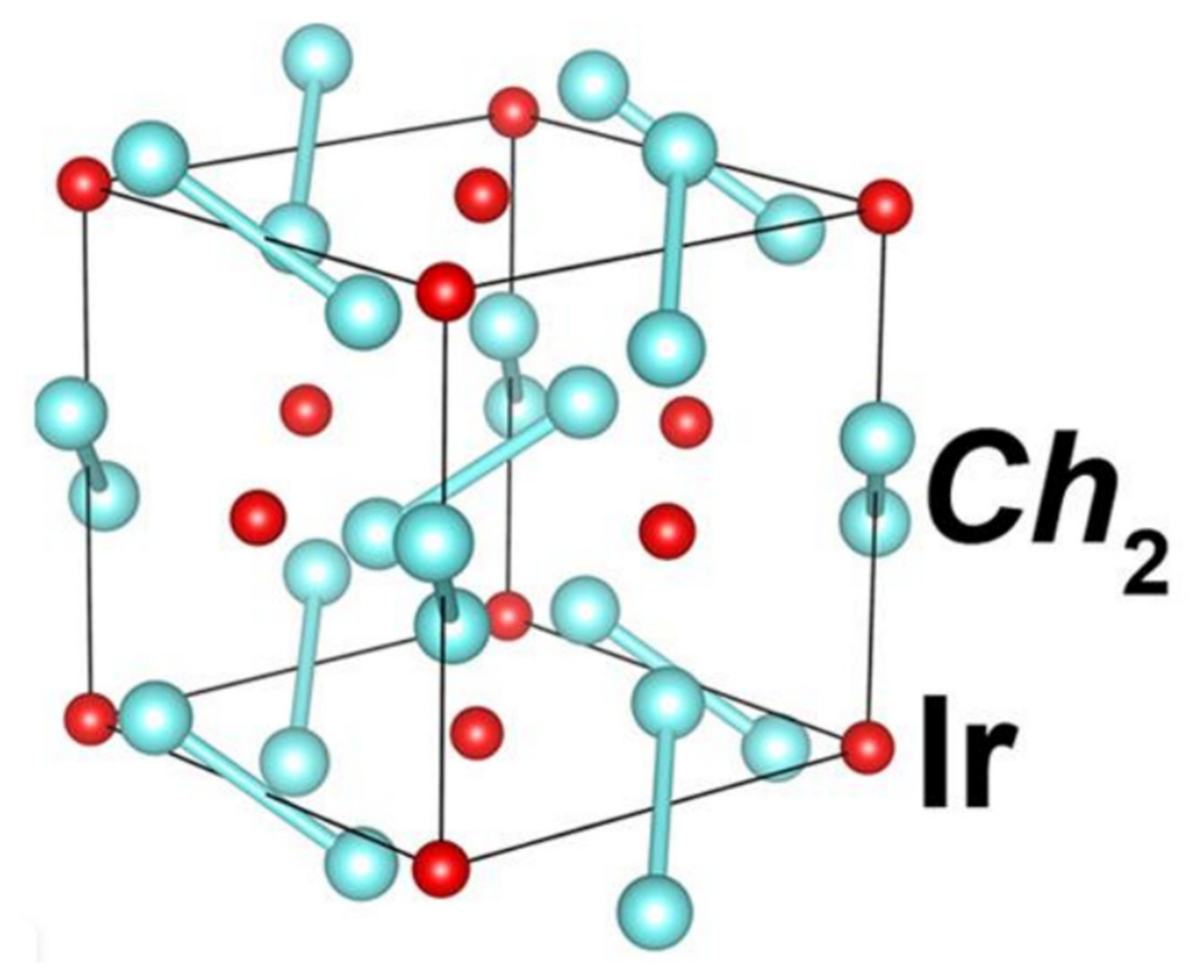
- Qi, Y.; Matsuishi, S.; Guo, J.; Mizoguchi, H.; Hosono, H. Superconductivity in Defective Pyrite-Type Iridium ChalcogenidesIrxCh2(Ch = Seand Te). Phys. Rev. Lett. 2012, 109, 217002.
- Gor’Kov, L.P.; Rashba, E.I. Superconducting 2D System with Lifted Spin Degeneracy: Mixed Singlet-Triplet State. Phys. Rev. Lett. 2001, 87, 037004.
- Frigeri, P.A.; Agterberg, D.F.; Sigrist, M. Spin susceptibility in superconductors without inversion symmetry. New J. Phys. 2004, 6, 115.
- Frigeri, P.A.; Agterberg, D.F.; Koga, A.; Sigrist, M. Superconductivity without Inversion Symmetry: MnSi versusCePt3Si. Phys. Rev. Lett. 2004, 92, 097001.
- Bauer, E.D.; Hilscher, G.; Michor, H.; Paul, C.; Scheidt, E.W.; Gribanov, A.V.; Seropegin, Y.; Noël, H.; Sigrist, M.; Rogl, P. Heavy Fermion Superconductivity and Magnetic Order in NoncentrosymmetricCePt3Si. Phys. Rev. Lett. 2004, 92, 027003.
- Settai, R.; Sugitani, I.; Okuda, Y.; Thamizhavel, A.; Nakashima, M.; Ōnuki, Y.; Harima, H. Pressure-induced superconductivity in CeCoGe3 without inversion symmetry. J. Magn. Magn. Mater. 2007, 310, 844–846.
- Kimura, N.; Ito, K.; Aoki, H.; Uji, S.; Terashima, T. Extremely High Upper Critical Magnetic Field of the Noncentrosymmetric Heavy Fermion SuperconductorCeRhSi3. Phys. Rev. Lett. 2007, 98, 197001.
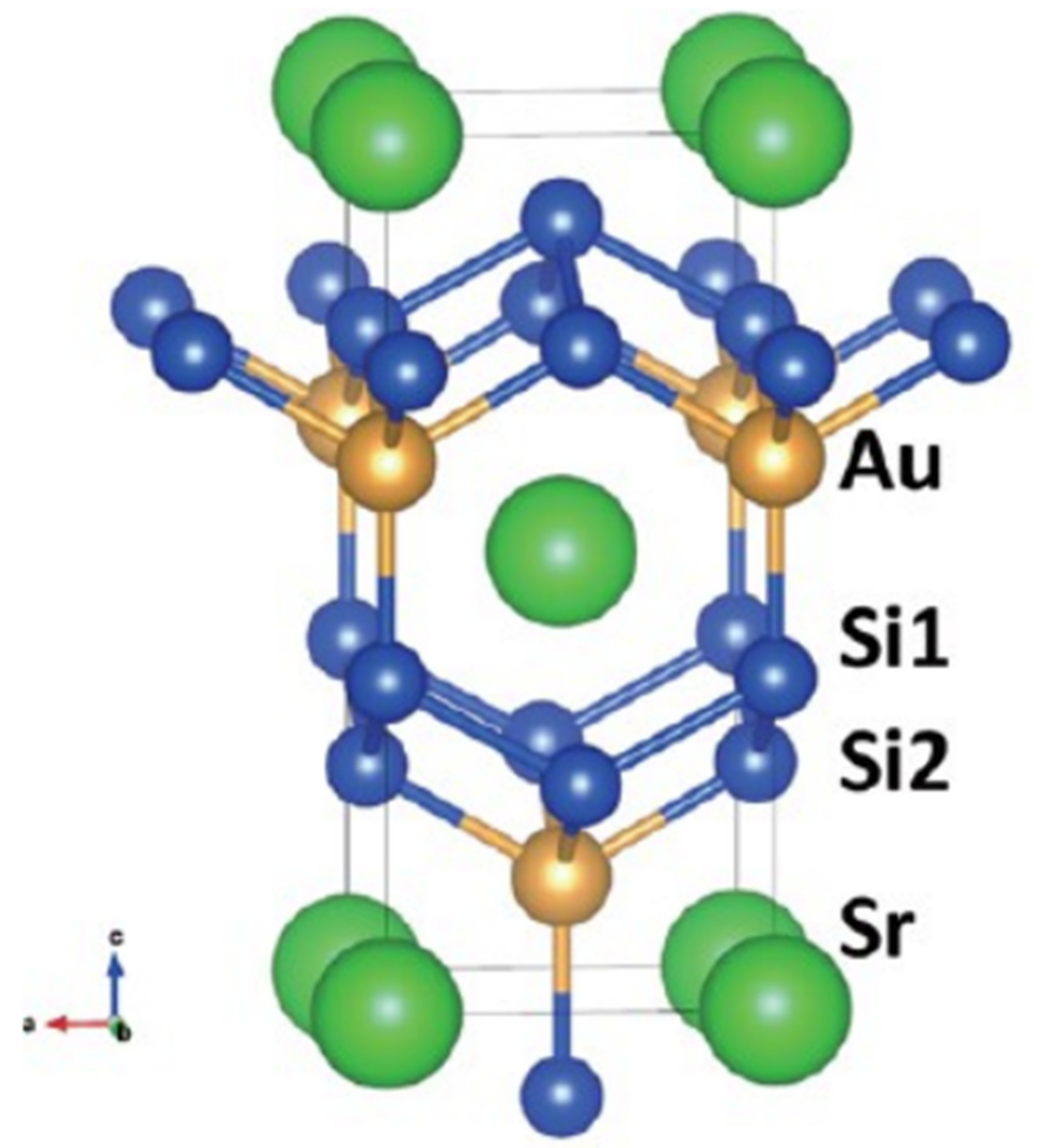
- Isobe, M.; Yoshida, H.; Kimoto, K.; Arai, M.; Takayama-Muromachi, E. SrAuSi3: A Noncentrosymmetric Superconductor. Cheminform 2014, 26, 2155–2165.
- Pyon, S.; Kudo, K.; Matsumura, J.-I.; Ishii, H.; Matsuo, G.; Nohara, M.; Hojo, H.; Oka, K.; Azuma, M.; Garlea, V.O.; et al. Superconductivity in Noncentrosymmetric Iridium Silicide Li2IrSi3. J. Phys. Soc. Jpn. 2014, 83, 1–5.
- McMillan, W.L. Transition Temperature of Strong-Coupled Superconductors. Phys. Rev. 1968, 167, 331–344.
- Dynes, R. McMillan’s equation and the Tc of superconductors. Solid State Commun. 1972, 10, 615–618.
- Zheng, X.; Zheng, J. Seeking high temperature superconductors in ambient from exemplary beryllium-based alloys. Solid State Commun. 2019, 306, 113769.
- Ryu, G.; Kim, S.W.; Mizoguchi, H.; Matsuishi, S.; Hosono, H. Superconductivity in a PbFCl-type pnictide: NbSiAs. Eur. Lett. 2012, 99, 27002.
- Mizoguchi, H.; Hosono, H. La2Sb, a layered superconductor with metal–metal bonds. Chem. Commun. 2011, 47, 3778–3780.
- Guo, J.; Yamaura, J.-I.; Lei, H.; Matsuishi, S.; Qi, Y.; Hosono, H. Superconductivity in Ban+2Ir4nGe12n+4(n = 1,2) with cage structure and softening of low-lying localized mode. Phys. Rev. B 2013, 88, 1–5.
- Ryu, G.; Kim, S.W.; Matsuishi, S.; Kawaji, H.; Hosono, H. Superconductivity in Nb4MSi (M = Ni, Co, and Fe) with a quasi-two-dimensional Nb network. Phys. Rev. B 2011, 84, 1–6.
- Li, Y.; Weng, Y.; Zhang, J.; Ding, J.; Zhu, Y.; Wang, Q.; Yang, Y.; Cheng, Y.; Zhang, Q.; Li, P.; et al. Observation of superconductivity in structure-selected Ti2O3 thin films. NPG Asia Mater. 2018, 10, 522–532.
- Jeong, S.; Matsuishi, S.; Lee, K.; Toda, Y.; Kim, S.W.; Hosono, H. Superconductivity of Ca2InN with a layered structure embedding an anionic indium chain array. Arxiv Prepr. 2014, arXiv:1403.1348.
- Arpaia, R.; Golubev, D.; Baghdadi, R.; Ciancio, R.; Dražić, G.; Orgiani, P.; Montemurro, D.; Bauch, T.; Lombardi, F. Transport properties of ultrathin YBa2Cu3O7−δ nanowires: A route to single-photon detection. Phys. Rev. B 2017, 96, 064525.
- Stepantsov, E.A.; Arpaia, R.; Lombardi, F. Growth of twin-free b-oriented YBa2Cu3O7 − x films. Crystallogr. Rep. 2015, 60, 393–396.
- Stepantsov, E.A.; Lombardi, F.; Winkler, D. Growth of YBa2Cu3O7 films with tilt of CuO planes to the surface on SrTiO3 crystals. Crystallogr. Rep. 2011, 56, 152–156.
- Kislinskii, J.; Zhao, B.-R.; Wu, P.-J.; Peng, Z.-Q.; Chen, Y.-F.; Yang, T.; Chen, L.; Sun, J.-J.; Xu, B.; Wu, F.; et al. YBa2Cu3O7 Bicrystal Josephson Junctions and dc SQUIDs. Chin. Phys. Lett. 1996, 13, 390–393.
- Carillo, F.; De Luca, G.M.; Montemurro, D.; Papari, G.P.; Salluzzo, M.; Stornaiuolo, D.; Tafuri, F.; Beltram, F. Coherent transport in extremely underdoped Nd1.2Ba1.8Cu3Oznanostructures. New J. Phys. 2012, 14, 1–10.
- Chaix, L.; Ghiringhelli, G.; Peng, Y.; Hashimoto, M.; Moritz, B.; Kummer, K.; Brookes, N.; He, Y.; Chen, S.; Ishida, S.; et al. Dispersive charge density wave excitations in Bi2Sr2CaCu2O8+δ. Nat. Phys. 2017, 13, 952–956.




