Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Estefan Monteiro Da Fonseca | -- | 2753 | 2022-06-27 15:08:52 | | | |
| 2 | Estefan Monteiro Da Fonseca | -1 word(s) | 2752 | 2022-06-27 16:58:09 | | | | |
| 3 | Catherine Yang | Meta information modification | 2752 | 2022-06-28 04:12:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Almeida, M.P.D.; Gaylarde, C.; Neto, J.A.B.; Neves, C.V.; Fonseca, E.M.D. Particulate and Floating Pollutants in the Oceans. Encyclopedia. Available online: https://encyclopedia.pub/entry/24515 (accessed on 14 January 2026).
Almeida MPD, Gaylarde C, Neto JAB, Neves CV, Fonseca EMD. Particulate and Floating Pollutants in the Oceans. Encyclopedia. Available at: https://encyclopedia.pub/entry/24515. Accessed January 14, 2026.
Almeida, Marcelo Pompermayer De, Christine Gaylarde, José Antônio Baptista Neto, Charles Vieira Neves, Estefan Monteiro Da Fonseca. "Particulate and Floating Pollutants in the Oceans" Encyclopedia, https://encyclopedia.pub/entry/24515 (accessed January 14, 2026).
Almeida, M.P.D., Gaylarde, C., Neto, J.A.B., Neves, C.V., & Fonseca, E.M.D. (2022, June 27). Particulate and Floating Pollutants in the Oceans. In Encyclopedia. https://encyclopedia.pub/entry/24515
Almeida, Marcelo Pompermayer De, et al. "Particulate and Floating Pollutants in the Oceans." Encyclopedia. Web. 27 June, 2022.
Copy Citation
The Earth’s oceans are the final resting place of anthropogenic residues, mainly plastics, metals, rubber, and fabrics, in order of decreasing abundance. After degradation resulted by UV rays atack, mechanical and chemical degradation, they tend to decant and deposit over the ocean floor. Most of these finaly assume fragmented or particulate forms, becoming colonized by marine microorganisms and later interacting with macroorganisms, leading to potential problems with marine life and the ecosystem. Rapid biodegradation of the polluting materials is still not possible, as a result of site contaminants atraction and accumulation and harmful by-products release.
Floating debris
Microplastic
Microrganisms
Biogeochemical Cycles
nanoplastic
1. Introduction
Global oceans face natural and anthropogenic challenges. As an answer, the United Nations has declared 2021 to 2030 the decade of restoration, promoting, among others, resilience to anthropogenic changes, especially in the oceans [1], which are considered the main sink of anthropogenic contaminants [2].
Detectable only with specific equipment, there is an abundant world of microorganisms inhabiting the oceanic ecosystem with a complexity and diversity that competes with all other forms of life on Earth. This group includes bacteria, viruses, fungi, and other microscopic organisms. Of all the living organisms in the ocean, about 50 percent of the biomass weight consists of microbes [3].
Microorganisms inhabit some of the environments considered extreme, such as scalding hydrothermal vents and even underground glacial lakes in Antarctica [4][5]. These were the first organisms to inhabit planet Earth, living in an anoxic environment in a pristine ocean [6]. As a result of them natural skills marine micro biota present fundamental importance to the balance of the ecossystems and resulting health of the Earth’s environments. They are responsible for the food and nutrient cycling that, in their absence, would not be bioaccessible in the ecosystems [7]. Many are also the guardians of ocean water balance and resulting in healthy ecosystems, recycling the ocean waste accumulations, and preventing the proliferation of opportunistic disease-causing beings.
A huge group of aquatic microorganisms shows the capacity to colonize surfaces, resulting in the formation of biofilms and the development of specialized processes within these structures [8][9]. As a survival mechanism, the surface colonization process in aquatic environments has a fundamental role for the microscopic species, since it represents greater access to nutritional resources, higher colony stability, and stronger specimen interactions, in a dynamic environment of low nutrient concentration. Sessile microorganisms (those attached to surfaces in biofilms) have advantages over the planktonic cells in their resistance to adverse conditions and antimicrobial substances, as well as possessing an increased metabolic rate.
Several types of surfaces with particular physicochemical and biological characteristics are offered by marine ecosystems; they include living organisms, among them animal and vegetal species. These substrata include many kinds of particles and aggregates, both inert and reactive mineral substrata. Aquatic solid substrates are ideal environments for important biogeochemical activities [10]. Surface colonization and biofilm production protect from predators, viruses, antibiotics, chemical toxins, and other deleterious environmental elements [11][12][13]. Polluting anthropogenic particles can serve as niches for the survival and replication of marine microorganisms.
2. Macrodebris
Marine litter, or macrodebris, is one of the sources of contaminants. Macrodebris not removed by other means will be broken down by mechanical, chemical, and biological activities in the oceans to add to the already present levels of polluting microparticles. Its vast distribution around the globe and long-life durability make marine litter a critical environmental issue [14][15]. This type of pollutant is present worldwide, from shallow water to the deep sea and from the poles to the equator. The most direct impact of marine litter on marine biota is the mechanical effect resulting from the entanglement of animals, which potentially harms their mobility, feeding, breathing, and reproduction capacities [16], affecting, most of the time, wandering species like fish, marine mammals, sea turtles and seabirds [17]. Sessile species are also potential targets; they are subject to mechanical impacts resulting from the movement of waste, which can affect their body structure [18][19].
In the deeper layers of the sea, larger-sized anthropogenic litter, which may be composed of plastic, metal, glass, rubber, and fabrics such as rope and clothing, is becoming increasingly common as human beings expand their oceanic activities.
These materials can become colonized by microorganisms within a few days [20], producing biofilms that differ not only according to the physical and chemical environment but also to the man-made substratum. It has been suggested that the main anthropogenic plastic litter in the oceans is associated with fishing activity [18][21][22][23][24]. 12.2 million tonnes of plastic are discarded into marine ecosystems per year, 80% being from coastal, 9.4% from fishing, and 4.9% from shipping origin [25][26].
3. Microplastics
The increased use of plastic materials over the last years has led to millions of tonnes per year of these recalcitrants being released into our seas. It is acknowledged that they are one of the most negatively significant anthropogenic impacts on our waters and are even found in the Arctic Sea, arriving there from as far away as the Asian coast [27]. They cause problems through their ingestion by aquatic life [28][29] and references therein. Microplastics also provide a surface on which pollutant molecules and potentially dangerous microorganisms can become concentrated and carried to other locations. Once the plastic becomes modified by oceanic organics, a completely new surface, much more attractive as a food source, is presented to living creatures.
There are five plastic accumulation zones in our oceans (Figure 1) [30], perhaps the best known of which is the Great Pacific Garbage Patch, in the north-central Pacific Ocean [31]. However, not all ocean gyres have been equally studied and, indeed, difficulties in standardizing sampling procedures make this a very problematic area [32].
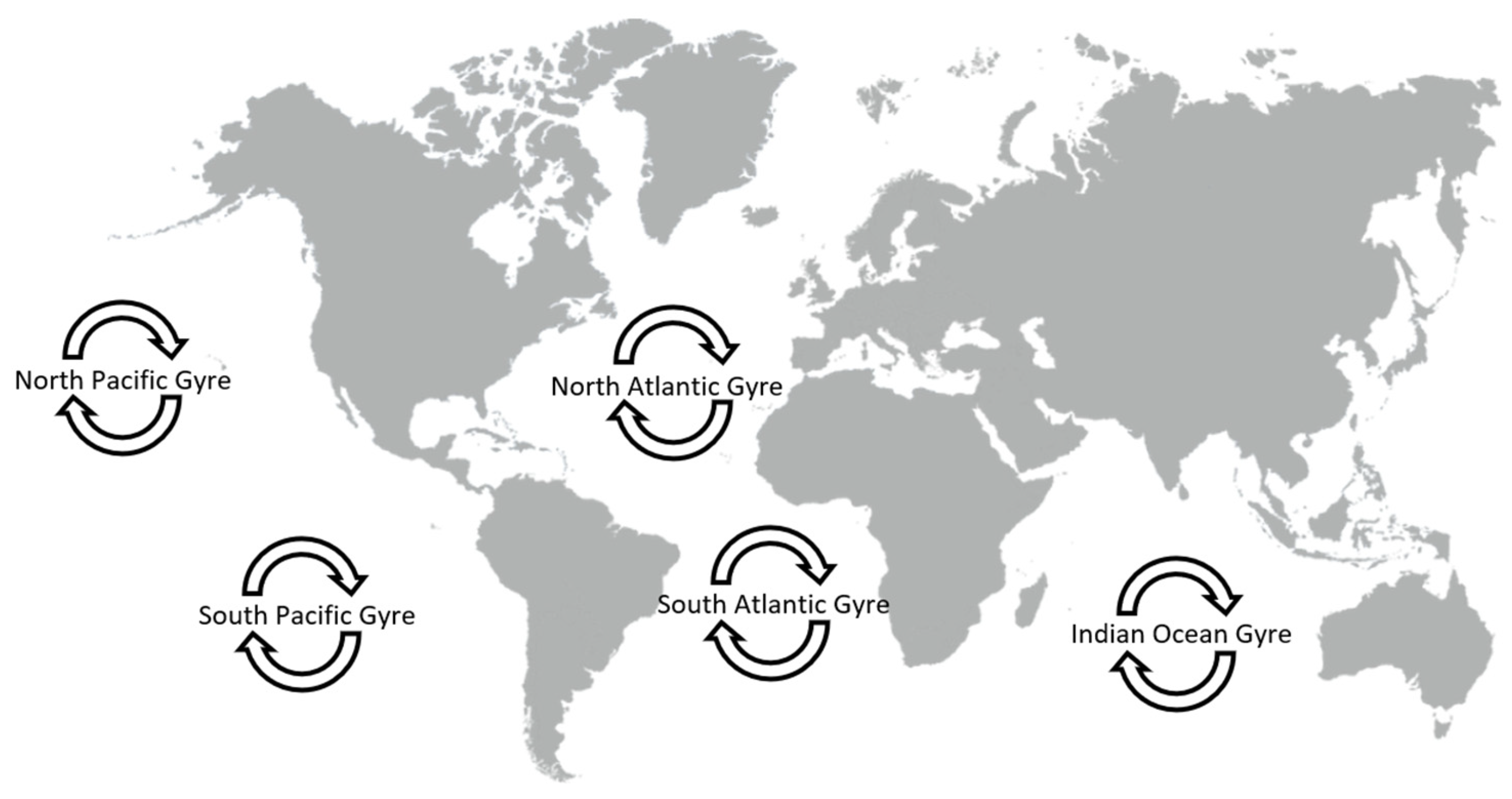
Figure 1. The five swirling ocean garbage patches are called “gyres”.
Initially, plastics are broken down in the seas into small fragments by abiotic forces such as u-v and wave action; when these fragments are less than 5 mm in size they are known as microplastics. They rapidly become covered by a thin film of organics present in the water and then by marine microorganisms (Figure 2), forming a surface population known as the plastisphere [33]. This contains a wide variety of prokaryotes and eukaryotes and the exact makeup may depend on the type of plastic, as well as the local environment [34][35], although it has been suggested that the plastisphere is only specific to plastic-type in conditions of low nutrients and low salinity [36]. A survey of plastisphere biofilms from around the world, and of various degrees of maturity, indicated that the most abundant phyla are Proteobacteria, Cyanobacteria, and Bacteroidetes [37].
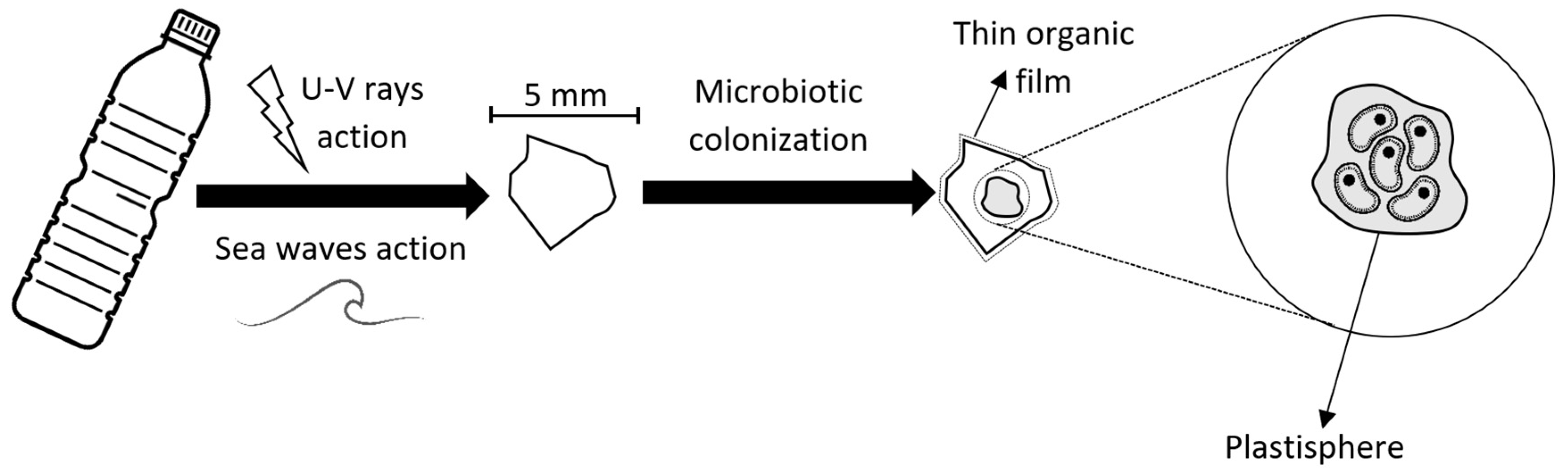
Figure 2. Microplastic colonization.
Colonized microplastics are carried on ocean currents and in spray and so transported to other regions, where the result may be prejudicial to the environment and its biota. It has been suggested that this may be one of the routes whereby metal- and antibiotic-resistant genes are spread around the world [38][39], but pathogenic microorganisms themselves may be transported in this way. For example, opportunistic invasive bacterial populations can enhance coral bleaching [40] and the biofilm in most plastic pools in the Bay of Brest has been shown to contain species related to the potential oyster pathogen, Vibrio splendidus [41].
4. Engineered Nanoparticles
Particles of up to 100 nm in diameter are known as nanoparticles. The European Union defines a suspension of nanoparticles as that in which 50% or more particles have one or more external dimensions of 1–100 nm. These suspensions exist in nature (e.g., nanoclays), but in the recent past, they have been manufactured for specific human purposes. Such engineered nanoparticles (ENPs) may be used, for example, in coatings, insulating and magnetic materials, and as antimicrobial additives, principally nanometal oxides, such as TiO2, which become increasingly antimicrobial under the action of UV In fact, metal and metal oxide nanoparticles are those most produced worldwide, with TiO2 having the highest produced mass [42], and many, along with silica nanoparticles, are used as antifouling materials for protection of marine and seawater-associated structures [43][44][45]. The antifouling materials can, of course, also affect the non-target biota in the sea, especially when liberated from the protected structures by sloughing or friction. Toxic effects of the principal metal oxide ENPs in the marine environment decrease in the order Au > Zn > Ag > Cu > Ti > Carbon60 [46].
At some stage in their lifetime, even if not utilized directly in the marine environment, ENPs will be released into their surroundings and end up entering the oceans, where they can exert negative effects on the biota [47][48], including inhibition of movement and metabolic processes, oxidative stress and dysfunctional DNA replication [49]. NanoZnO particles ranging from 30 nm to 2 μm have been shown to be highly toxic to flounder cells in culture and zebrafish embryos [50]. ENPs can readily pass from terrestrial waters into the ocean and thence into the marine food web [51]. It has been suggested that the main effects of ENPs on coastal marine life forms will be in sediments [52], where they have been shown to have toxic effects on foraminifera [53].
There has been little empirical study of the fate of ENPs in the aquatic environment [54], although it has been suggested that they can cause significant harm to the marine ecosystem [55] and references therein and that they have significant toxic effects on marine phytoplankton [56]. The release of silver NPs has been shown to alter the functioning of the marine food web by hampering important viral and bacterial processes [57]. In a mesocosm experiment, the addition of silver NPs, even at a low dose, affected planktonic communities, especially reducing the growth of the cyanobacterium Synechococcus. Viral auxiliary metabolic genes involved in cyanobacterial photosynthesis were also decreased.
It has, however, been suggested recently that ENPs may not be found at sufficiently high concentrations in the natural environment to pose a current problem [58]. More data on the effects and fate of nanoparticles released into the environment are necessary. Life cycle and ecological risk assessments of ENPs in our oceans are essential to stimulate remediation processes and protect the marine environment.
5. Metallic Particles
Mining of polymetallic nodules, found on the surface of abyssal plains at around 4000 m depth, results in the release into the benthos of sediment plumes and nodule debris. These can be rich in Mn, Ni, Cu, and Co [59]. Fazey and Ryan [60] examined the aerobically grown bacteria present on the surface of nodules and in the overlying sediment, identifying Halomonas aquamarina, H. meridiana, and Erythrobacter citreus in both, but the genera Arthrobacter, Kocuria, Loktanella, Marinobacter and Pseudoalteromonas only in sediment within 4 cm of the nodule surface. Cho et al. [61] confirmed that the microbiome of nodules differs from the microbial population in the surrounding sediment, but their use of NGS technology led to the detection of a different set of bacteria and Archaea. Thaumarchaeota were found in both sediment and nodule, Mn-oxidizing bacteria (Hyphomycrobium, Aurantimonas, and Marinobacter) were predominant in nodules, and Idiomarina, Erythrobacter, and Sulfitobacter in sediments. Gillard et al. [59], based on their analyses, suggest the use of standard cultivation techniques for monitoring plume propagation; indicator organisms for sediment would be Diezia maris and Pseudoalteromonas shioyasakiensis, for nodules Rhodococcus erythropolis and water Marinobacter flavimaris.
Much of the iron found in aerosols over the oceans is anthropogenic in origin, resulting from the burning of fossil and biofuels and fires on land (biomass burning), a situation that is probably mirrored by zinc [62]. Conway et al. [63], using iron-isotope ratios, showed that deposition of anthropogenic Fe could reach almost 100% of the total Fe near highly populated areas. Their model suggested that this effect would be greatest in the Southern and Pacific Oceans, and this was echoed by Hamilton et al. in 2020 [64]. Much of the iron, and, indeed, many metals found in marine particles may be linked to the presence of microorganisms that produce metal-chelating siderophores. Chuang et al. [65] found that hydroxamate siderophores comprised a large part of the sinking particles (“marine snow” q.v.) collected in the Sargasso Sea. One of the important ecological functions of the siderophores produced by microorganisms is the release of iron from sinking particles to supply dissolved iron to the water column [66]. The export of iron from hydrothermal vents in the Southern East Pacific has likewise been linked to particles containing microorganisms [67].
Pollution by mercuric ions is a potential risk to human health, principally through the consumption of fish [68]. The main anthropogenic source of this metal is artisanal and small-scale gold mining, followed by the burning of fossil fuels [69]. The metal is converted to toxic methylmercury and dimethylmercury by microbial activity in the seas [70] and is largely associated with marine particulate matter [71]. The latter authors identified the sulfate-reducing bacterium, Desulfovibrio desulfuricans, as important for the uptake or exchange of Hg2+ in anaerobic environments. Marine Group II (MGII) archaeal genes associated with assimilatory sulfate reduction have been detected, along with MGII genes involved in surface adhesion, in samples collected from around the world during the Tara Oceans’ circumnavigation trip [72]. The authors suggested that archaea MGII could be implicated in the degradation of marine particles, a more positive role for microbial biofilms in our oceans.
Marine microorganisms are also important in the production of metallic compounds. The mineral barite (or baryte), used principally in drilling muds, is produced in the oceans by barium binding initially to phosphate groups in bacterial cells or EPS; the thus concentrated barium is then converted in the marine environment to barite [73].
6. Sinking Particles (“Marine Snow”) and Pollution
Marine snow is considered to be composed of heterogeneous agglomerates of living and dead organic matter of >500 um in size, formed by the attachment of organisms to the so-called “transparent exopolymer particles” (TEPs) that consist mainly of acidic polysaccharides previously produced by phytoplankton and heterotrophic prokaryotes [74] and references therein. The particles contain diverse groups of eukaryotes, which may somewhat resemble, but certainly do not equal, the plankton in the local environment [75]. They have a highly variable composition and there are fundamental differences in particle composition between oligotrophic and eutrophic environments [75]. The microbial taxa associated with the particles are very different from those in the surrounding seawater and may contain increased oil degraders in oil-polluted environments [76][77] or methylmercury genes in saline waters in the North Sea [78]. Hence the particles may be “hot spots” for the degradative activity of surrounding pollutants [79].
Such sinking particles differ from floating particles in the oceans in carrying a changing population of prokaryotic species. Duret et al. [80] identified the prokaryotic populations on both types of particles in the Scotia Sea (Southern Ocean) and suggested that r-strategists, with generalized metabolic activities and rapid substrate consumption, were better adapted to sinking particles, with their changing environment, while K-strategists, specialized for complex organic material degradation, were better adapted to the more stable environment of semi-labile floating particles. So, for instance, pseudomonads and Rhodobacteriales were enriched on sinking particles, Flavobacteriales on floating. Datta et al. [81] had previously shown, using model polysaccharide particles, that the attached bacterial communities underwent rapid metabolic successions, driven by the environment. They suggested that there are 3 phases of colonization: attachment of a highly diverse community, selection of specific metabolic activities by the environment (reducing diversity) and replacement by secondary consumers, metabolizing the products of the second phase cells, and increasing diversity somewhat once more. Liu et al. [82], investigating differences between the two types of particles at low and high pressures in the New Britain Trench, Solomon Sea (Pacific), found that, although there were differences in prokaryotic populations on floating and sinking particles, similar groups participated in the degradation of diatom debris.
Even if similar organisms are involved in degradation, the physical act of sinking, whereby water flows past the particle surface, increases the rate of biodegradation simply by aiding the removal of the degradation products, driving the reaction to the right. This increase in microbiodegradation in sinking, as opposed to static, particles was elegantly demonstrated in a mathematical model developed by Alcolombri et al. [83].
The presence of eukaryotes in marine snow has been less frequently investigated. Bochdansky et al. [84] showed the presence of a fungal biomass equal to that of prokaryotes in bathypelagic particles from the North Atlantic and Arctic seas. Fungi and labyrinthulomycetes (the latter mainly labyrinthulids and thraustochytrids) dominated the biomass. These eukaryotes are tolerant of low temperatures and high pressures and were considered to be potentially important biodegraders in the particles. Schultz et al. [85], using metaproteomics and functional analyses of marine particles, showed that eukaryotes were more abundant in the particles than in the surrounding seawater. Those detected in particles were phytoplankton, Oomycetes, and Fungi. Greater amounts of viral proteins were also found in the particles. They reported rather small differences between bacterial proteins on particles and in the planktonic phase. The relative abundance of eukaryotes and viruses confirmed the results of López-Pérez et al. [86], who investigated the coastal waters off Alicante, Spain. They also found that there was an overrepresentation in the particle-associated microbiome of alpha, delta, and gamma proteobacteria, bacteroidetes (Flavobacteria), Planktomycetes, and Actinobacteria.
References
- Coleman, M.A.; Wood, G.; Filbee-Dexter, K.; Minne, A.J.P.; Goold, H.D.; Verges, A.; Marzinelli, E.M.; Steinberg, P.D.; Wernberg, T. Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 2020, 7, 237.
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayami, M.; Qafoku, N.P.; Yang, Y.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299.
- Bar-On, Y.M.; Milo, R. The biomass composition of the oceans: A blueprint of our blue planet. Cell 2019, 179, 1451–1454.
- Ando, N.; Barquera, B.; Bartlett, D.H.; Boyd, E.; Burnim, A.A.; Byer, A.S.; Colman, D.; Gillilan, R.E.; Gruebele, M.; Makhatadze, G.; et al. The molecular basis for life in extreme environments. Annu. Rev. Biophys 2021, 50, 343–372.
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial Diversity in Extreme Marine Habitats and Their Biomolecules. Microorganisms 2017, 5, 25.
- Henderson, J.; Salem, H. CHAPTER 1: The atmosphere: Its developmental history and contributions to microbial evolution and habitat. In Aerobiology: The Toxicology of Airborne Pathogens and Toxins; RSC Publishing: London, UK, 2016; pp. 1–41. ISBN 978-1-84973-791-3.
- Isobe, K.; Ohte, N. Ecological perspectives on microbes involved in N-cycling. Microbes Environ. 2014, 29, 4–16.
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745.
- Pierce, E.C.; Dutton, R.J. Putting microbial interactions back into community contexts. Curr. Opin. Microbiol. 2022, 65, 56–63.
- Roukaerts, A.; Deman, F.; Van der Linden, F.; Carnat, G.; Bratkic, A.; Moreau, S.; Dehairs, F.; Delille, B.; Tison, J.-L.; Fripiat, F. The biogeochemical role of a microbial biofilm in sea ice: Antarctic landfast sea ice as a case study. Elem. Sci. Anthr. 2021, 9, 00134.
- Gadkari, J.; Bhattacharya, S.; Shrivastav, A. Importance and applications of biofilm in microbe-assisted bioremediation. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Nertherland, 2022; pp. 153–173.
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471.
- Tan, D.; Svenningsen, S.L.; Middelboe, M. Quorum sensing determines the choice of antiphage defense strategy in Vibrio anguillarum. mBio 2015, 6, e00627-15.
- McIlgorm, A.; Raubenheimer, K.; McIlgorm, D.E.; Nichols, R. The cost of marine litter damage to the global marine economy: Insights from the Asia-Pacific into prevention and the cost of inaction. Mar. Pollut. Bull. 2022, 174, 113167.
- Baptista Neto, J.A.; Gaylarde, C.; da Fonseca, E.M. Microplastics: A pelagic habitat for microorganisms and invertebrates. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2021.
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349.
- Richardson, K.; Asmutis-Silvia, R.; Drinkwin, J.; Gilardi, K.V.K.; Giskes, I.; Jones, G.; O’Brian, K.; Pragnell-Raasch, H.; Ludwig, L.; Antonelis, K.; et al. Building evidence around ghost gear: Global trends and analysis for sustainable solutions at scale. Mar. Pollut. Bull. 2019, 138, 222–229.
- Angiolillo, M. Debris in Deep Water. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 14; pp. 251–268.
- Angiolillo, M.; Fortibuoni, T. Impacts of marine litter on Mediterranean reef systems: From shallow to deep waters. Front. Mar. Sci. 2020, 7, 826.
- Lee, J.-W.; Nam, J.-H.; Kim, Y.-H.; Lee, K.-H.; Lee, D.-H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 2008, 46, 174–182.
- Caruso, G. Microplastics in marine environments: Possible interactions with the microbial assemblage. J. Pollut. Eff. Cont. 2015, 3, e111.
- Lee, D.-I.; Cho, H.-S.; Jeong, S.-B. Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Estuar Coast. Shelf Sci. 2006, 70, 187–194.
- Melli, V.; Angiolillo, M.; Ronchi, F.; Canese, S.; Giovanardi, O.; Querin, S.; Fortibuoni, T. The first assessment of marine debris in a Site of Community Importance in the north-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 2017, 114, 821–830.
- Watters, D.L.; Yoklavich, M.M.; Love, M.S.; Schroeder, D.M. Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 2010, 60, 131–138.
- Sherrington, C. Plastics in the Marine Environment; Eunomia Research & Consulting Ltd.: Bristol, UK, 2016; p. 16.
- Wei, C.-L.; Rowe, G.T.; Nunnally, C.C.; Wicksten, M.K. Anthropogenic “Litter” and macrophyte detritus in the deep Northern Gulf of Mexico. Mar. Pollut. Bull. 2012, 64, 966–973.
- Halsband, C.; Herzke, D. Plastic litter in the European Arctic: What do we know? Emerg. Contam. 2019, 5, 308–318.
- Setala, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83.
- Rogers, K.L.; Carreres-Calabuig, J.A.; Gorokhova, E.; Posth, N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. 2020, 5, 18–36.
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244.
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132.
- Montoto-Martínez, T.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Pump-underway ship intake: An unexploited opportunity for Marine Strategy Framework Directive (MSFD) microplastic monitoring needs on coastal and oceanic waters. PLoS ONE 2020, 15, e0232744.
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.S. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146.
- Dudek, K.L.; Cruz, B.N.; Polidoro, B.; Neuer, S. Microbial colonization of microplastics in the Caribbean Sea. Limnol. Oceanogr. Lett. 2020, 5, 5–17.
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The Plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859.
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709.
- Schlundt, C.; Welch, M.J.L.; Knochel, A.M.; Zettler, E.; Amarel-Zettler, L. Spatial structure in the “Plastisphere”: Molecular resources for imaging microscopic communities on plastic marine debris. Mol. Ecol. Resour. 2019, 20, 620–634.
- Moore, R.E.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR) and marine plastics: Can food packaging litter act as a dispersal mechanism for AMR in oceanic environments? Mar. Pollut. Bull. 2020, 150, 110702.
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Int. 2019, 123, 79–86.
- Zhou, J.; Lin, Z.-J.; Cai, Z.-H.; Zeng, Y.-H.; Zhu, J.-M.; Dhu, X.-P. Opportunistic bacteria use quorum sensing to disturb coral symbiotic communities and mediate the occurrence of coral bleaching. Environ. Microbiol. 2020, 22, 1944–1962.
- Frère, L.; Maignien, L.; Chalopin, M. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242 Pt A, 614–625.
- Dedman, C.J.; King, A.M.; Christie-Oleza, J.A.; Davies, G.L. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ. Sci. Nano 2021, 8, 1236–1255.
- Abioye, O.P.; Loto, C.A.; Fayomi, O.S.I. Evaluation of Anti-biofouling Progresses in Marine Application. J. Bio-Tribo-Corros. 2019, 5, 22.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417.
- Wang, D.; Xu, J.; Yang, J.; Zhou, S. Preparation and synergistic antifouling effect of self-renewable coatings containing quaternary ammonium-functionalized SiO2 nanoparticles. J. Colloid. Interface Sci. 2020, 563, 261–271.
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyer, C.; Camacho-Chab, J.C.; Pereanez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Herit. Sci. 2018, 6, 52.
- Falfushynska, H.; Sokolova, I.; Stoika, R. Uptake, biodistribution, and mechanisms of toxicity of metal-containing nanoparticles in aquatic invertebrates and vertebrates. In Biomedical Nanomaterials; Springer: Cham, Switzerland, 2022; pp. 227–263.
- Zha, S.J.; Rong, J.H.; Guan, X.F.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248.
- Solano, R.; Patiño-Ruiz, D.; Tejeda-Benitez, L.; Herrera, A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: A review on the applications, nanotoxicological effects, and risk control strategies. Environ. Sci. Pollut. Res. 2021, 28, 16962–16981.
- Han, L.; Zhai, Y.; Liu, Y.; Hao, L.; Guo, H. Comparison of the in vitro and in vivo toxic effects of three sizes of zinc oxide (ZnO) particles using flounder gill (FG) cells and zebrafish embryos. J. Ocean Univ. China 2017, 16, 93–106.
- Ferry, J.; Craig, P.; Hexel, C.; Sisco, P.; Frey, R.; Pennington, P.L.; Fulton, M.H.; Scott, I.G.; Decho, A.W.; Kashiwada, S.; et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol. 2009, 4, 441–444.
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the risk of engineered nanomaterials in the environment: Development and application of the nanoFate model. Environ. Sci. Technol. 2017, 51, 5541–5551.
- Ciacci, C.; Grimmelpont, M.V.; Corsi, I.; Bergami, E.; Curzi, D.; Burini, D.; Bouchet, V.M.P.; Ambrogini, P.; Gobbi, P.; Ujiie, Y.; et al. Nanoparticle-biological interactions in a marine benthic foraminifer. Sci. Rep. 2019, 9, 19441.
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; von der Kammer, F.; Kuhlbusch, T.A.J.; Nickel, C.; Quick, J.T.K.; Renkerg, M.; Koelmans, A.A. A Review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134.
- Chiu, M.; Khan, Z.A.; Garcia, S.G. Effect of engineered nanoparticles on exopolymeric substances release from marine phytoplankton. Nanoscale Res. Lett. 2017, 12, 620.
- Sendra, M.; Moreno, I.; Blasco, J. Toxicity of metal and metal oxide engineered nanoparticles to phytoplankton. In Ecotoxicity of Nanoparticles in Aquatic Systems; Blasco, J., Corsi, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1351657550/9781351657556.
- Tsiola, A.; Toncelli, C.; Fodelianakis, S.; Michaud, G.; Bucheli, T.; Gavriilidou, A.; Kagiorgi, M.; Kalantzi, I.; Knauer, K.; Kotulas, G.; et al. Low-dose addition of silver nanoparticles stresses marine plankton communities. Environ. Sci. Nano 2018, 5, 1965–1980.
- Zhao, J.; Lin, M.; Wang, Z.; Cao, X.; Xing, B. Engineered nanomaterials in the environment: Are they safe? Crit. Rev. Environ. Sci. Technol. 2021, 51, 1443–1478.
- Gillard, B.; Chatzievangelou, D.; Thomsen, L.; Ullrich, M.S. Heavy-metal-resistant microorganisms in deep-sea sediments disturbed by mining activity: An application toward the development of experimental in vitro systems. Front. Mar. Sci. 2019, 6, 432.
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360.
- Cho, H.; Kim, K.; Son, S.K.; Le, A.D.; Kagiri, A.; Ramos, J.; Tsai, S.M.; Drobenaire, H.W.; Santschi, P.H.; Quigg, A. Fine-scale microbial communities associated with manganese nodules in deep-sea sediment of the Korea Deep Ocean Study Area in the Northeast Equatorial Pacific. Ocean Sci. J. 2018, 53, 337–353.
- Lemaitre, N.; de Souza, G.F.; Archer, C.; Wang, R.-M.; Planquette, H.; Sarthou, G.; Vance, D. Pervasive sources of isotopically light zinc in the North Atlantic Ocean. Earth Planet Sci. Lett. 2020, 539, 116216.
- Conway, T.M.; Hamilton, D.S.; Shelley, R.U.; Aguilar-Islas, A.M.; Landing, W.M.; Mahowald, M.N.; John, S.G. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 2019, 10, 2628.
- Hamilton, D.S.; Moore, J.K.; Arneth, A.; Bond, T.C.; Carslaw, K.S.; Hantson, S.; Ito, A.; Kaplan, J.O.; Lindsay, K.; Nieradzik, L.P.; et al. Impact of changes to the atmospheric soluble iron deposition flux on ocean biogeochemical cycles in the Anthropocene. Glob. Biogeochem. Cycles 2020, 34, e2019GB006448.
- Chuang, C.-Y.; Santschi, P.H.; Ho, Y.-F.; Conte, M.; Guo, L.; Schumann, D.; Ayranov, M.; Li, Y.-h. Biopolymers as major carrier phases and redox regulators of Th, Pa, Pb, Po, and Be in settling particles from the Atlantic Ocean. Mar. Chem. 2013, 15, 131–143.
- Boyd, P.; Ellwood, M.; Tagliabue, A.; Twining, B.S. Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nat. Geosci. 2017, 10, 167–173.
- Hoffman, C.L.; Nicholas, S.L.; Ohnemus, D.C.; Fitzsimmons, J.; Sherrell, R.; German, C.; Heller, M.; Lee, J.-M.; Lam, P.; Toner, B.M.; et al. Near-field iron and carbon chemistry of non-buoyant hydrothermal plume particles, Southern East Pacific Rise 15°S. Mar. Chem. 2018, 201, 183–197.
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105.
- Arctic Monitoring and Assessment Programme (AMAP). United Nations Environment Programme (UNEP) Global Mercury Assessment: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013.
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in marine and oceanic waters—A review. Water Air Soil Pollut. 2016, 227, 371.
- Zhang, L.; Wu, S.; Zhao, L.; Liu, X.; Pierce, E.M.; Gu, B. Mercury sorption and desorption on organo-mineral particulates as a source for microbial methylation. Environ. Sci. Technol. 2019, 53, 2426–2433.
- Pereira, O.; Hochart, C.; Auguet, J.C.; Debroas, D.; Galand, P.E. Genomic ecology of Marine Group II, the most common marine planktonic Archaea across the surface ocean. MicrobiologyOpen 2019, 8, e852.
- Martinez-Ruiz, F.; Paytan, A.; Gonzalez-Muñoz, M.T.; Jroundi, F.; Abad, M.M.; Lam, P.J.; Kastner, M. Barite formation in the ocean: Origin of amorphous and crystalline precipitates. Chem. Geol. 2019, 511, 441–451.
- Zamanillo, M.; Ortega-Retuerta, E.; Nunes, S.; Rodriguez-Ros, P.; Dall’Osto, M.; Estrada, M.; Sala, M.M.; Simo, R. Main drivers of transparent exopolymer particle distribution across the surface Atlantic Ocean. Biogeosciences 2019, 16, 733–749.
- Lundgreen, R.B.C.; Jaspers, C.; Traving, S.J.; Ayala, D.J.; Lombard, F.; Grossart, H.-P.; Nielsen, T.G.; Munk, P.; Riemann, L. Eukaryotic and cyanobacterial communities associated with marine snow particles in the oligotrophic Sargasso Sea. Sci. Rep. 2019, 9, 8891.
- Arnosti, C.; Ziervogel, K.; Yang, T.; Teske, A. Oil-derived marine 817 aggregates–hot spots of polysaccharide degradation by specialized bacterial 818 communities. Deep-Sea Res. PT II 2016, 129, 179–186.
- Duran Suja, L.; Summers, S.; Gutierrez, T. Role of EPS, Dispersant and Nutrients on the Microbial Response and MOS Formation in the Subarctic Northeast Atlantic. Front. Microbiol. 2017, 8, 676.
- Capo, E.; Bravo, A.G.; Soerensen, A.L.; Bertilsson, S.; Pinhassi, J.; Feng, C.; Andersson, A.F.; Buck, M.; Bjorn, E. Marine snow as a habitat for microbial mercury methylators in the Baltic Sea. bioRxiv 2020, 3, 975987.
- Achberger, A.M.; Doyle, S.M.; Mills, M.I.; Holmes II, C.P.; Quigg, A.; Sylvan, J.B. Bacteria-Oil Microaggregates Are an Important Mechanism for Hydrocarbon Degradation in the Marine Water Column. mSystems 2021, 6, e01105-21.
- Duret, M.T.; Lampitt, R.S.; Lam, P. Prokaryotic niche partitioning between suspended and sinking marine particles. Environ. Microbiol. Rep. 2019, 11, 386–400.
- Datta, M.; Sliwerska, E.; Gore, J.; Polz, M.F.; Cordero, O.X. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat. Commun. 2016, 7, 11965.
- Liu, Y.; Fang, J.; Jia, Z.; Chen, S.; Zhang, L.; Gao, W. DNA stable-isotope probing reveals potential key players for microbial decomposition and degradation of diatom-derived marine particulate matter. MicrobiologyOpen 2020, 9, e1013.
- Alcolombri, U.; Peaudecerf, F.J.; Fernandez, V.I.; Behrendt, L.; Lee, K.S.; Stocker, R. Sinking enhances the degradation of organic particles by marine bacteria. Nat. Geosci. 2021, 14, 775–780.
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373.
- Schultz, D.; Zühlke, D.; Bernhardt, J.; Francis, T.B.; Albrecht, D.; Hirschfeld, C.; Markert, S.; Riedel, K. An optimized metaproteomics protocol for a holistic taxonomic and functional characterization of microbial communities from marine particles. Environ. Microbiol. Rep. 2020, 12, 367–376.
- López-Pérez, M.; Kimes, N.E.; Haro-Moreno, J.M.; Rodriguez-Valera, F. Not all particles are equal: The selective enrichment of particle-associated bacteria from the Mediterranean sea. Front. Microbiol. 2016, 7, 996.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
966
Entry Collection:
Environmental Sciences
Revisions:
3 times
(View History)
Update Date:
28 Jun 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




