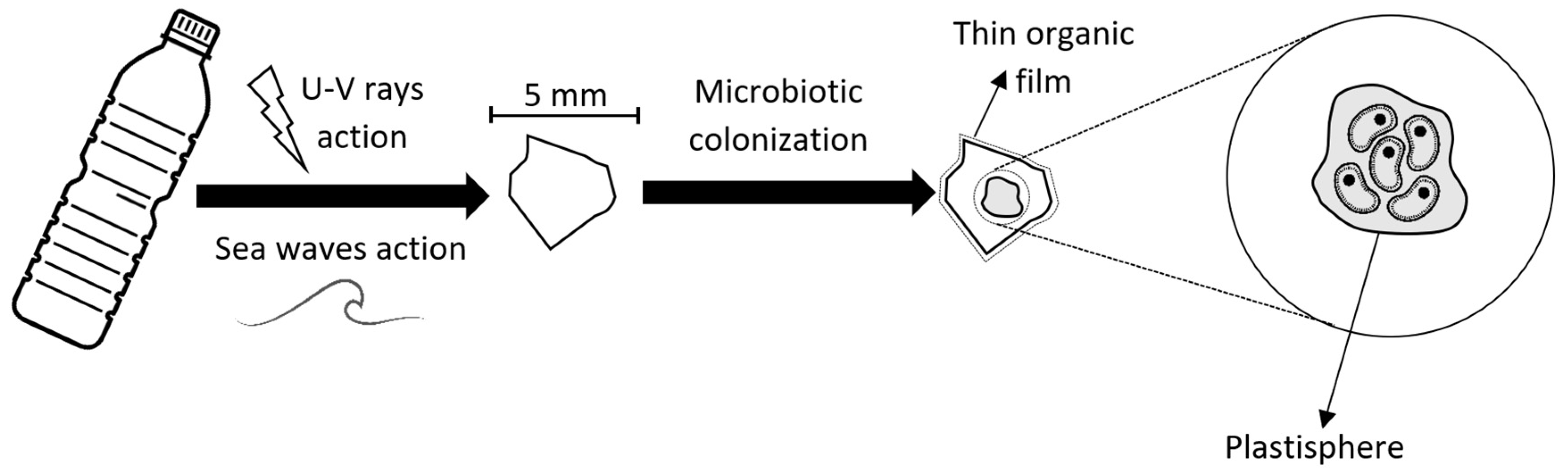
The Earth’s oceans are the final resting place of anthropogenic residues, mainly plastics, metals, rubber, and fabrics, in order of decreasing abundance. After degradation resulted by UV rays atack, mechanical and chemical degradation, they tend to decant and deposit over the ocean floor. Most of these finaly assume fragmented or particulate forms, becoming colonized by marine microorganisms and later interacting with macroorganisms, leading to potential problems with marine life and the ecosystem. Rapid biodegradation of the polluting materials is still not possible, as a result of site contaminants atraction and accumulation and harmful by-products release.
- Floating debris
- Microplastic
- Microrganisms
- Biogeochemical Cycles
- nanoplastic
1. Introduction
2. Macrodebris
3. Microplastics
4. Engineered Nanoparticles
5. Metallic Particles
6. Sinking Particles (“Marine Snow”) and Pollution
This entry is adapted from the peer-reviewed paper 10.3390/micro2020017
References
- Coleman, M.A.; Wood, G.; Filbee-Dexter, K.; Minne, A.J.P.; Goold, H.D.; Verges, A.; Marzinelli, E.M.; Steinberg, P.D.; Wernberg, T. Restore or redefine: Future trajectories for restoration. Front. Mar. Sci. 2020, 7, 237.
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayami, M.; Qafoku, N.P.; Yang, Y.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299.
- Bar-On, Y.M.; Milo, R. The biomass composition of the oceans: A blueprint of our blue planet. Cell 2019, 179, 1451–1454.
- Ando, N.; Barquera, B.; Bartlett, D.H.; Boyd, E.; Burnim, A.A.; Byer, A.S.; Colman, D.; Gillilan, R.E.; Gruebele, M.; Makhatadze, G.; et al. The molecular basis for life in extreme environments. Annu. Rev. Biophys 2021, 50, 343–372.
- Poli, A.; Finore, I.; Romano, I.; Gioiello, A.; Lama, L.; Nicolaus, B. Microbial Diversity in Extreme Marine Habitats and Their Biomolecules. Microorganisms 2017, 5, 25.
- Henderson, J.; Salem, H. CHAPTER 1: The atmosphere: Its developmental history and contributions to microbial evolution and habitat. In Aerobiology: The Toxicology of Airborne Pathogens and Toxins; RSC Publishing: London, UK, 2016; pp. 1–41. ISBN 978-1-84973-791-3.
- Isobe, K.; Ohte, N. Ecological perspectives on microbes involved in N-cycling. Microbes Environ. 2014, 29, 4–16.
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745.
- Pierce, E.C.; Dutton, R.J. Putting microbial interactions back into community contexts. Curr. Opin. Microbiol. 2022, 65, 56–63.
- Roukaerts, A.; Deman, F.; Van der Linden, F.; Carnat, G.; Bratkic, A.; Moreau, S.; Dehairs, F.; Delille, B.; Tison, J.-L.; Fripiat, F. The biogeochemical role of a microbial biofilm in sea ice: Antarctic landfast sea ice as a case study. Elem. Sci. Anthr. 2021, 9, 00134.
- Gadkari, J.; Bhattacharya, S.; Shrivastav, A. Importance and applications of biofilm in microbe-assisted bioremediation. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Nertherland, 2022; pp. 153–173.
- Serra, D.O.; Hengge, R. Stress responses go three dimensional—the spatial order of physiological differentiation in bacterial macrocolony biofilms. Environ. Microbiol. 2014, 16, 1455–1471.
- Tan, D.; Svenningsen, S.L.; Middelboe, M. Quorum sensing determines the choice of antiphage defense strategy in Vibrio anguillarum. mBio 2015, 6, e00627-15.
- McIlgorm, A.; Raubenheimer, K.; McIlgorm, D.E.; Nichols, R. The cost of marine litter damage to the global marine economy: Insights from the Asia-Pacific into prevention and the cost of inaction. Mar. Pollut. Bull. 2022, 174, 113167.
- Baptista Neto, J.A.; Gaylarde, C.; da Fonseca, E.M. Microplastics: A pelagic habitat for microorganisms and invertebrates. In Handbook of Microplastics in the Environment; Rocha-Santos, T., Costa, M., Mouneyrac, C., Eds.; Springer: Cham, Switzerland, 2021.
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349.
- Richardson, K.; Asmutis-Silvia, R.; Drinkwin, J.; Gilardi, K.V.K.; Giskes, I.; Jones, G.; O’Brian, K.; Pragnell-Raasch, H.; Ludwig, L.; Antonelis, K.; et al. Building evidence around ghost gear: Global trends and analysis for sustainable solutions at scale. Mar. Pollut. Bull. 2019, 138, 222–229.
- Angiolillo, M. Debris in Deep Water. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; Chapter 14; pp. 251–268.
- Angiolillo, M.; Fortibuoni, T. Impacts of marine litter on Mediterranean reef systems: From shallow to deep waters. Front. Mar. Sci. 2020, 7, 826.
- Lee, J.-W.; Nam, J.-H.; Kim, Y.-H.; Lee, K.-H.; Lee, D.-H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. J. Microbiol. 2008, 46, 174–182.
- Caruso, G. Microplastics in marine environments: Possible interactions with the microbial assemblage. J. Pollut. Eff. Cont. 2015, 3, e111.
- Lee, D.-I.; Cho, H.-S.; Jeong, S.-B. Distribution characteristics of marine litter on the sea bed of the East China Sea and the South Sea of Korea. Estuar Coast. Shelf Sci. 2006, 70, 187–194.
- Melli, V.; Angiolillo, M.; Ronchi, F.; Canese, S.; Giovanardi, O.; Querin, S.; Fortibuoni, T. The first assessment of marine debris in a Site of Community Importance in the north-western Adriatic Sea (Mediterranean Sea). Mar. Pollut. Bull. 2017, 114, 821–830.
- Watters, D.L.; Yoklavich, M.M.; Love, M.S.; Schroeder, D.M. Assessing marine debris in deep seafloor habitats off California. Mar. Pollut. Bull. 2010, 60, 131–138.
- Sherrington, C. Plastics in the Marine Environment; Eunomia Research & Consulting Ltd.: Bristol, UK, 2016; p. 16.
- Wei, C.-L.; Rowe, G.T.; Nunnally, C.C.; Wicksten, M.K. Anthropogenic “Litter” and macrophyte detritus in the deep Northern Gulf of Mexico. Mar. Pollut. Bull. 2012, 64, 966–973.
- Halsband, C.; Herzke, D. Plastic litter in the European Arctic: What do we know? Emerg. Contam. 2019, 5, 308–318.
- Setala, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83.
- Rogers, K.L.; Carreres-Calabuig, J.A.; Gorokhova, E.; Posth, N.R. Micro-by-micro interactions: How microorganisms influence the fate of marine microplastics. Limnol. Oceanogr. 2020, 5, 18–36.
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernandez-Leon, S.; Palma, A.T.; Navarro, S.; Garcia-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244.
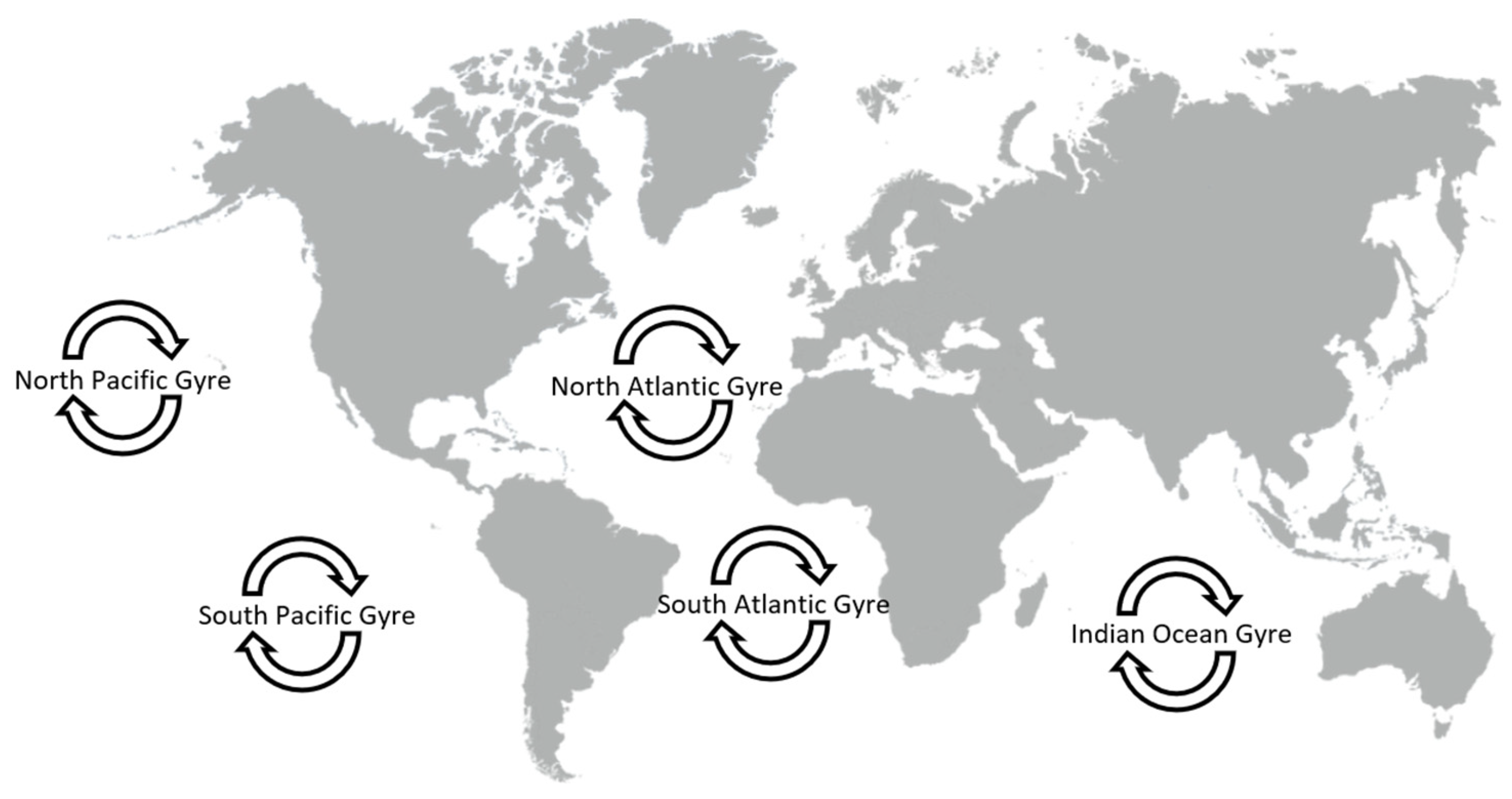
- Carson, H.S.; Nerheim, M.S.; Carroll, K.A.; Eriksen, M. The plastic-associated microorganisms of the North Pacific Gyre. Mar. Pollut. Bull. 2013, 75, 126–132.
- Montoto-Martínez, T.; Hernández-Brito, J.J.; Gelado-Caballero, M.D. Pump-underway ship intake: An unexploited opportunity for Marine Strategy Framework Directive (MSFD) microplastic monitoring needs on coastal and oceanic waters. PLoS ONE 2020, 15, e0232744.
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.S. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146.
- Dudek, K.L.; Cruz, B.N.; Polidoro, B.; Neuer, S. Microbial colonization of microplastics in the Caribbean Sea. Limnol. Oceanogr. Lett. 2020, 5, 5–17.
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The Plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859.
- Oberbeckmann, S.; Kreikemeyer, B.; Labrenz, M. Environmental factors support the formation of specific bacterial assemblages on microplastics. Front. Microbiol. 2018, 8, 2709.
- Schlundt, C.; Welch, M.J.L.; Knochel, A.M.; Zettler, E.; Amarel-Zettler, L. Spatial structure in the “Plastisphere”: Molecular resources for imaging microscopic communities on plastic marine debris. Mol. Ecol. Resour. 2019, 20, 620–634.
- Moore, R.E.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR) and marine plastics: Can food packaging litter act as a dispersal mechanism for AMR in oceanic environments? Mar. Pollut. Bull. 2020, 150, 110702.
- Yang, Y.; Liu, G.; Song, W.; Ye, C.; Lin, H.; Li, Z.; Liu, W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environ. Int. 2019, 123, 79–86.
- Zhou, J.; Lin, Z.-J.; Cai, Z.-H.; Zeng, Y.-H.; Zhu, J.-M.; Dhu, X.-P. Opportunistic bacteria use quorum sensing to disturb coral symbiotic communities and mediate the occurrence of coral bleaching. Environ. Microbiol. 2020, 22, 1944–1962.
- Frère, L.; Maignien, L.; Chalopin, M. Microplastic bacterial communities in the Bay of Brest: Influence of polymer type and size. Environ. Pollut. 2018, 242 Pt A, 614–625.
- Dedman, C.J.; King, A.M.; Christie-Oleza, J.A.; Davies, G.L. Environmentally relevant concentrations of titanium dioxide nanoparticles pose negligible risk to marine microbes. Environ. Sci. Nano 2021, 8, 1236–1255.
- Abioye, O.P.; Loto, C.A.; Fayomi, O.S.I. Evaluation of Anti-biofouling Progresses in Marine Application. J. Bio-Tribo-Corros. 2019, 5, 22.
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417.
- Wang, D.; Xu, J.; Yang, J.; Zhou, S. Preparation and synergistic antifouling effect of self-renewable coatings containing quaternary ammonium-functionalized SiO2 nanoparticles. J. Colloid. Interface Sci. 2020, 563, 261–271.
- Reyes-Estebanez, M.; Ortega-Morales, B.O.; Chan-Bacab, M.; Granados-Echegoyer, C.; Camacho-Chab, J.C.; Pereanez-Sacarias, J.E.; Gaylarde, C. Antimicrobial engineered nanoparticles in the built cultural heritage context and their ecotoxicological impact on animals and plants: A brief review. Herit. Sci. 2018, 6, 52.
- Falfushynska, H.; Sokolova, I.; Stoika, R. Uptake, biodistribution, and mechanisms of toxicity of metal-containing nanoparticles in aquatic invertebrates and vertebrates. In Biomedical Nanomaterials; Springer: Cham, Switzerland, 2022; pp. 227–263.
- Zha, S.J.; Rong, J.H.; Guan, X.F.; Tang, Y.; Han, Y.; Liu, G. Immunotoxicity of four nanoparticles to a marine bivalve species, Tegillarca granosa. J. Hazard. Mater. 2019, 377, 237–248.
- Solano, R.; Patiño-Ruiz, D.; Tejeda-Benitez, L.; Herrera, A. Metal- and metal/oxide-based engineered nanoparticles and nanostructures: A review on the applications, nanotoxicological effects, and risk control strategies. Environ. Sci. Pollut. Res. 2021, 28, 16962–16981.
- Han, L.; Zhai, Y.; Liu, Y.; Hao, L.; Guo, H. Comparison of the in vitro and in vivo toxic effects of three sizes of zinc oxide (ZnO) particles using flounder gill (FG) cells and zebrafish embryos. J. Ocean Univ. China 2017, 16, 93–106.
- Ferry, J.; Craig, P.; Hexel, C.; Sisco, P.; Frey, R.; Pennington, P.L.; Fulton, M.H.; Scott, I.G.; Decho, A.W.; Kashiwada, S.; et al. Transfer of gold nanoparticles from the water column to the estuarine food web. Nat. Nanotechnol. 2009, 4, 441–444.
- Garner, K.L.; Suh, S.; Keller, A.A. Assessing the risk of engineered nanomaterials in the environment: Development and application of the nanoFate model. Environ. Sci. Technol. 2017, 51, 5541–5551.
- Ciacci, C.; Grimmelpont, M.V.; Corsi, I.; Bergami, E.; Curzi, D.; Burini, D.; Bouchet, V.M.P.; Ambrogini, P.; Gobbi, P.; Ujiie, Y.; et al. Nanoparticle-biological interactions in a marine benthic foraminifer. Sci. Rep. 2019, 9, 19441.
- Peijnenburg, W.J.G.M.; Baalousha, M.; Chen, J.; Chaudry, Q.; von der Kammer, F.; Kuhlbusch, T.A.J.; Nickel, C.; Quick, J.T.K.; Renkerg, M.; Koelmans, A.A. A Review of the properties and processes determining the fate of engineered nanomaterials in the aquatic environment. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2084–2134.
- Chiu, M.; Khan, Z.A.; Garcia, S.G. Effect of engineered nanoparticles on exopolymeric substances release from marine phytoplankton. Nanoscale Res. Lett. 2017, 12, 620.
- Sendra, M.; Moreno, I.; Blasco, J. Toxicity of metal and metal oxide engineered nanoparticles to phytoplankton. In Ecotoxicity of Nanoparticles in Aquatic Systems; Blasco, J., Corsi, I., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 1351657550/9781351657556.
- Tsiola, A.; Toncelli, C.; Fodelianakis, S.; Michaud, G.; Bucheli, T.; Gavriilidou, A.; Kagiorgi, M.; Kalantzi, I.; Knauer, K.; Kotulas, G.; et al. Low-dose addition of silver nanoparticles stresses marine plankton communities. Environ. Sci. Nano 2018, 5, 1965–1980.
- Zhao, J.; Lin, M.; Wang, Z.; Cao, X.; Xing, B. Engineered nanomaterials in the environment: Are they safe? Crit. Rev. Environ. Sci. Technol. 2021, 51, 1443–1478.
- Gillard, B.; Chatzievangelou, D.; Thomsen, L.; Ullrich, M.S. Heavy-metal-resistant microorganisms in deep-sea sediments disturbed by mining activity: An application toward the development of experimental in vitro systems. Front. Mar. Sci. 2019, 6, 432.
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ. Pollut. 2016, 210, 354–360.
- Cho, H.; Kim, K.; Son, S.K.; Le, A.D.; Kagiri, A.; Ramos, J.; Tsai, S.M.; Drobenaire, H.W.; Santschi, P.H.; Quigg, A. Fine-scale microbial communities associated with manganese nodules in deep-sea sediment of the Korea Deep Ocean Study Area in the Northeast Equatorial Pacific. Ocean Sci. J. 2018, 53, 337–353.
- Lemaitre, N.; de Souza, G.F.; Archer, C.; Wang, R.-M.; Planquette, H.; Sarthou, G.; Vance, D. Pervasive sources of isotopically light zinc in the North Atlantic Ocean. Earth Planet Sci. Lett. 2020, 539, 116216.
- Conway, T.M.; Hamilton, D.S.; Shelley, R.U.; Aguilar-Islas, A.M.; Landing, W.M.; Mahowald, M.N.; John, S.G. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 2019, 10, 2628.
- Hamilton, D.S.; Moore, J.K.; Arneth, A.; Bond, T.C.; Carslaw, K.S.; Hantson, S.; Ito, A.; Kaplan, J.O.; Lindsay, K.; Nieradzik, L.P.; et al. Impact of changes to the atmospheric soluble iron deposition flux on ocean biogeochemical cycles in the Anthropocene. Glob. Biogeochem. Cycles 2020, 34, e2019GB006448.
- Chuang, C.-Y.; Santschi, P.H.; Ho, Y.-F.; Conte, M.; Guo, L.; Schumann, D.; Ayranov, M.; Li, Y.-h. Biopolymers as major carrier phases and redox regulators of Th, Pa, Pb, Po, and Be in settling particles from the Atlantic Ocean. Mar. Chem. 2013, 15, 131–143.
- Boyd, P.; Ellwood, M.; Tagliabue, A.; Twining, B.S. Biotic and abiotic retention, recycling and remineralization of metals in the ocean. Nat. Geosci. 2017, 10, 167–173.
- Hoffman, C.L.; Nicholas, S.L.; Ohnemus, D.C.; Fitzsimmons, J.; Sherrell, R.; German, C.; Heller, M.; Lee, J.-M.; Lam, P.; Toner, B.M.; et al. Near-field iron and carbon chemistry of non-buoyant hydrothermal plume particles, Southern East Pacific Rise 15°S. Mar. Chem. 2018, 201, 183–197.
- Sundseth, K.; Pacyna, J.M.; Pacyna, E.G.; Pirrone, N.; Thorne, R.J. Global sources and pathways of mercury in the context of human health. Int. J. Environ. Res. Public Health 2017, 14, 105.
- Arctic Monitoring and Assessment Programme (AMAP). United Nations Environment Programme (UNEP) Global Mercury Assessment: Sources, Emissions, Releases and Environmental Transport; UNEP Chemicals Branch: Geneva, Switzerland, 2013.
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in marine and oceanic waters—A review. Water Air Soil Pollut. 2016, 227, 371.
- Zhang, L.; Wu, S.; Zhao, L.; Liu, X.; Pierce, E.M.; Gu, B. Mercury sorption and desorption on organo-mineral particulates as a source for microbial methylation. Environ. Sci. Technol. 2019, 53, 2426–2433.
- Pereira, O.; Hochart, C.; Auguet, J.C.; Debroas, D.; Galand, P.E. Genomic ecology of Marine Group II, the most common marine planktonic Archaea across the surface ocean. MicrobiologyOpen 2019, 8, e852.
- Martinez-Ruiz, F.; Paytan, A.; Gonzalez-Muñoz, M.T.; Jroundi, F.; Abad, M.M.; Lam, P.J.; Kastner, M. Barite formation in the ocean: Origin of amorphous and crystalline precipitates. Chem. Geol. 2019, 511, 441–451.
- Zamanillo, M.; Ortega-Retuerta, E.; Nunes, S.; Rodriguez-Ros, P.; Dall’Osto, M.; Estrada, M.; Sala, M.M.; Simo, R. Main drivers of transparent exopolymer particle distribution across the surface Atlantic Ocean. Biogeosciences 2019, 16, 733–749.
- Lundgreen, R.B.C.; Jaspers, C.; Traving, S.J.; Ayala, D.J.; Lombard, F.; Grossart, H.-P.; Nielsen, T.G.; Munk, P.; Riemann, L. Eukaryotic and cyanobacterial communities associated with marine snow particles in the oligotrophic Sargasso Sea. Sci. Rep. 2019, 9, 8891.
- Arnosti, C.; Ziervogel, K.; Yang, T.; Teske, A. Oil-derived marine 817 aggregates–hot spots of polysaccharide degradation by specialized bacterial 818 communities. Deep-Sea Res. PT II 2016, 129, 179–186.
- Duran Suja, L.; Summers, S.; Gutierrez, T. Role of EPS, Dispersant and Nutrients on the Microbial Response and MOS Formation in the Subarctic Northeast Atlantic. Front. Microbiol. 2017, 8, 676.
- Capo, E.; Bravo, A.G.; Soerensen, A.L.; Bertilsson, S.; Pinhassi, J.; Feng, C.; Andersson, A.F.; Buck, M.; Bjorn, E. Marine snow as a habitat for microbial mercury methylators in the Baltic Sea. bioRxiv 2020, 3, 975987.
- Achberger, A.M.; Doyle, S.M.; Mills, M.I.; Holmes II, C.P.; Quigg, A.; Sylvan, J.B. Bacteria-Oil Microaggregates Are an Important Mechanism for Hydrocarbon Degradation in the Marine Water Column. mSystems 2021, 6, e01105-21.
- Duret, M.T.; Lampitt, R.S.; Lam, P. Prokaryotic niche partitioning between suspended and sinking marine particles. Environ. Microbiol. Rep. 2019, 11, 386–400.
- Datta, M.; Sliwerska, E.; Gore, J.; Polz, M.F.; Cordero, O.X. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat. Commun. 2016, 7, 11965.
- Liu, Y.; Fang, J.; Jia, Z.; Chen, S.; Zhang, L.; Gao, W. DNA stable-isotope probing reveals potential key players for microbial decomposition and degradation of diatom-derived marine particulate matter. MicrobiologyOpen 2020, 9, e1013.
- Alcolombri, U.; Peaudecerf, F.J.; Fernandez, V.I.; Behrendt, L.; Lee, K.S.; Stocker, R. Sinking enhances the degradation of organic particles by marine bacteria. Nat. Geosci. 2021, 14, 775–780.
- Bochdansky, A.B.; Clouse, M.A.; Herndl, G.J. Eukaryotic microbes, principally fungi and labyrinthulomycetes, dominate biomass on bathypelagic marine snow. ISME J. 2017, 11, 362–373.
- Schultz, D.; Zühlke, D.; Bernhardt, J.; Francis, T.B.; Albrecht, D.; Hirschfeld, C.; Markert, S.; Riedel, K. An optimized metaproteomics protocol for a holistic taxonomic and functional characterization of microbial communities from marine particles. Environ. Microbiol. Rep. 2020, 12, 367–376.
- López-Pérez, M.; Kimes, N.E.; Haro-Moreno, J.M.; Rodriguez-Valera, F. Not all particles are equal: The selective enrichment of particle-associated bacteria from the Mediterranean sea. Front. Microbiol. 2016, 7, 996.
