Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ya-Chu Tsai | -- | 2115 | 2022-05-19 09:24:34 | | | |
| 2 | Peter Tang | Meta information modification | 2115 | 2022-05-19 10:57:02 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tsai, Y.; Tsai, T. Overlapping Features of Psoriasis and Atopic Dermatitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/23110 (accessed on 08 February 2026).
Tsai Y, Tsai T. Overlapping Features of Psoriasis and Atopic Dermatitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/23110. Accessed February 08, 2026.
Tsai, Ya-Chu, Tsen-Fang Tsai. "Overlapping Features of Psoriasis and Atopic Dermatitis" Encyclopedia, https://encyclopedia.pub/entry/23110 (accessed February 08, 2026).
Tsai, Y., & Tsai, T. (2022, May 19). Overlapping Features of Psoriasis and Atopic Dermatitis. In Encyclopedia. https://encyclopedia.pub/entry/23110
Tsai, Ya-Chu and Tsen-Fang Tsai. "Overlapping Features of Psoriasis and Atopic Dermatitis." Encyclopedia. Web. 19 May, 2022.
Copy Citation
Psoriasis (PSO) and atopic dermatitis (AD) were once considered to be mutually exclusive diseases, but gradually regarded as a spectrum of disease. Shared genetic loci of both diseases were noted in some populations, including Chinese. Shared immunopathogenesis involving Th17, Th1, Th22 cells, or even IL-13 was found in certain stages or phenotypes.
atopic dermatitis
psoriasis
overlap
concomitant
paradoxical
psoriasis dermatitis
1. Introduction
Psoriasis (PSO) and atopic dermatitis (AD) are common inflammatory skin diseases with distinct clinical manifestations [1]. The prevalence of PSO is 2% in Caucasians and 0.2% in Asian regions [2][3]. AD has a much higher prevalence, especially in children (up to 20%) [4][5]. PSO and AD each have their own typical involvement areas. PSO tends to occur on the scalp and extensor skin, while AD varies with age, i.e., the extensor side of extremities and face in infancy; flexure side and hands in adolescents and adults.
Historically, opposing immunopathogenic mechanisms, Th2 and Th1, had been proposed for these diseases. Besides, different RNA transcriptomes [6] and barrier profiles have been revealed [7]. With regard to the disease course, PSO has a peak onset around 20–30 years of age, and most patients continue to suffer throughout life, while AD usually begins in early childhood with improvement before adolescence. In fact, patients with PSO were reported to have a 25-fold lower prevalence of AD [8]. Thus, the concurrence of PSO and AD was once considered to be very rare.
However, a recent study has shown a directional association between PSO and AD [9]. There are shared genetic profiles, immune pathways, pathologic changes, and comorbidities for the diseases. Indeed, retrospective and case studies about concomitant AD and PSO have been increasingly reported [10][11][12][13]. They may occur as diseases with overlapping features or coexisting diseases on different body regions in the same individual (Figure 1). Conventional oral immunosuppressive therapy and phototherapy can treat both AD and PSO, but biologic agents targeting only specific T-cells or cytokines are often ineffective for concurrent diseases, and might even induce transformation from one disease to the other.
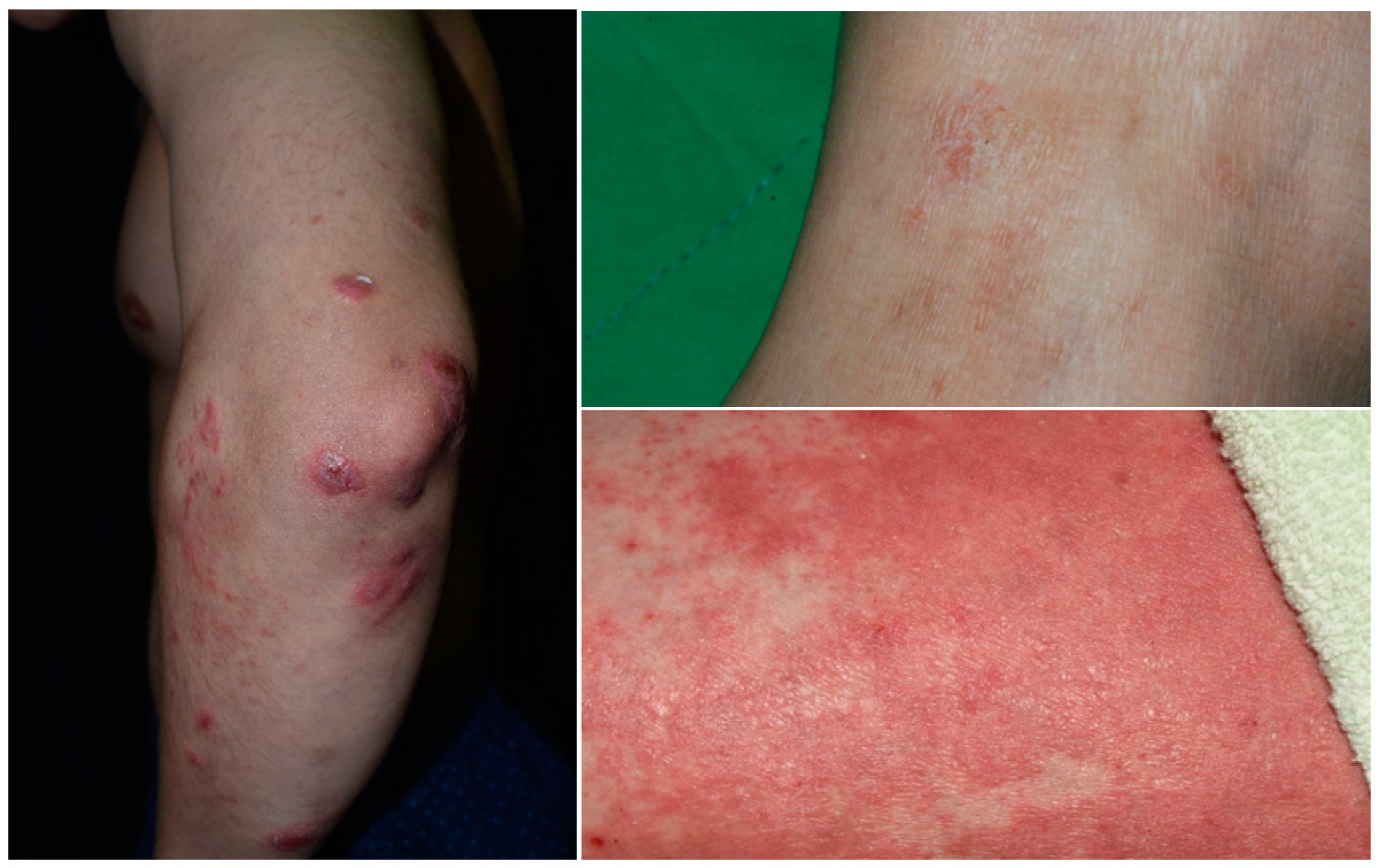
Figure 1. Psoriasis with overlapping features of eczema.
2. Shared Genetic Background
Both PSO and AD have a strong genetic background, with family segregation and higher disease risks in monozygotic twins compared with dizygotic twins. Dozens of genetic loci have been discovered in the diseases, respectively, which correspond to the genetic heterogeneity. The most frequent susceptibility gene locus of PSO is HLA-Cw*0602 (on PSORS1 6p21), while null mutations of the FLG gene is the strongest genetic risk for developing AD [1][2][3][5].
In terms of genes on the overlapping chromosomal loci of the diseases, contrasting results were revealed in comparative studies. Most studies indicated that the epidermal differentiation complex (EDC) on chromosome 1q21.3 contains FLG gene mutations for AD, which has no relation to PSO [14][15][16]. Likewise, the late cornified envelope (LCE) genes 3B/3C deletion within the EDC for PSO is not related to AD [14][17]. However, variants of FLG mutation were reported to confer a risk of developing psoriasis in Taiwanese and Chinese populations [18][19].
Another shared region on the genome is chromosome 5q31.1-q33.1, where IL-13 has shown associations with both AD and PSO [14]. Previous data indicated that IL-13 was a signature cytokine of AD, more important than IL-4 [20]. Baurecht et al. proposed the opposing risk alleles at this shared locus (chromosome 5q31.1, the Th2 cytokine control area) of the diseases [14]. However, there was also evidence to support the relationship between IL-13 and PSO/psoriatic arthritis [21][22]. In brief, most genetic analyses are in favor of AD and PSO as opposing diseases, but overlapping loci or shared cytokines have been noted, although their influence on diseases remains unclear.
3. Shared Immunopathogenesis
3.1. PSO
Genetic predisposition and environmental triggers interact to induce PSO. Stimulated keratinocytes release antimicrobial peptide LL37, which further amplifies toll-like receptor 9 signaling on plasmacytoid dendritic cells (pDC). Activated pDCs produce interferon (IFN)-α, which enhances myeloid dendritic cell (mDC) maturation. IFNα is also related to the differentiation of Th1 and Th17 and the production of IFNγ and IL-17. Myeloid DCs can be activated via LL37 as well. After activating, mDCs migrate to draining lymph nodes to release TNFα and IL-23. IL-23 modulates Th17 cell proliferation and maturation. Th17 secretes IL-17 and IL-22 combined with TNFα and IFNγ to induce keratinocyte hyperproliferation and undifferentiation. IL-17 is mainly secreted by Th17 and type 3 innate lymphoid cells (ILC3) in psoriasis. Overall, the TNFα-IL23-Th17-IL17A/F pathway is the hallmark of plaque-type psoriasis. On the other hand, pustular psoriasis involves the mutation of the IL-36 receptor antagonist secreted by keratinocytes [1][2][3].
3.2. AD
A combination of genetic background, epidermal barrier defects, microbiome imbalance, and immune dysregulation contribute to AD. Although the Th2 pathway is the main driving pathway of AD, the multipolar involvement of immune axes leads to various phenotypes and severities. The addition of environmental stress on epidermal barrier defects activates dendritic cells and type II cytokine-related response. Stimulated Th2 cells release IL-4, IL-13, IL-31, and keratinocytes produce IL-33 and TSLP, driving further inflammation and barrier dysfunction. Type 2 innate lymphoid cells (ILC2) generate IL-5 and IL-13 cytokines, which recruit eosinophils and Th2 cells. The accompanied elevation of IL-22, mainly produced by Th22 and Th17, inhibits keratinocyte differentiation and induces epidermal hyperplasia. Moreover, Th1 plays a role in chronic AD, while the Th17 axis relates to the Asian or pediatric types of AD. However, trials regarding the Th17 pathway failed to achieve adequate efficacy for moderate to severe AD, so its clinical significance still needs further evaluation in AD. In terms of atopic march (allergic march), it might be related to chronic IgE sensitization by IL-4 and IL-13 [1][4][5][23].
3.3. PSO and AD
PSO is mainly an IL23-Th17-IL17 disease, while AD is Th2 skewing associated with IL-4 and IL-13. Nevertheless, Asian, pediatric, and intrinsic types of AD involve Th17 as well [1][23][24]. Analysis of PSO susceptibility genes identified an odds ratio of 1.18 increase in IL-4/IL-13 signaling loci [25]. Furthermore, both diseases involve Th1 and Th22. However, IL-22 might not be essential to PSO and AD, since the blocking of IL-22 did not prove its efficacy in PSO treatment [26] and was only moderately effective for AD in a phase 2a trial [27]. A following study in AD was suspended, accordingly. Although IL-22 levels were increased in both diseases, it might not be the culprit, but an innocent bystander (Figure 2).
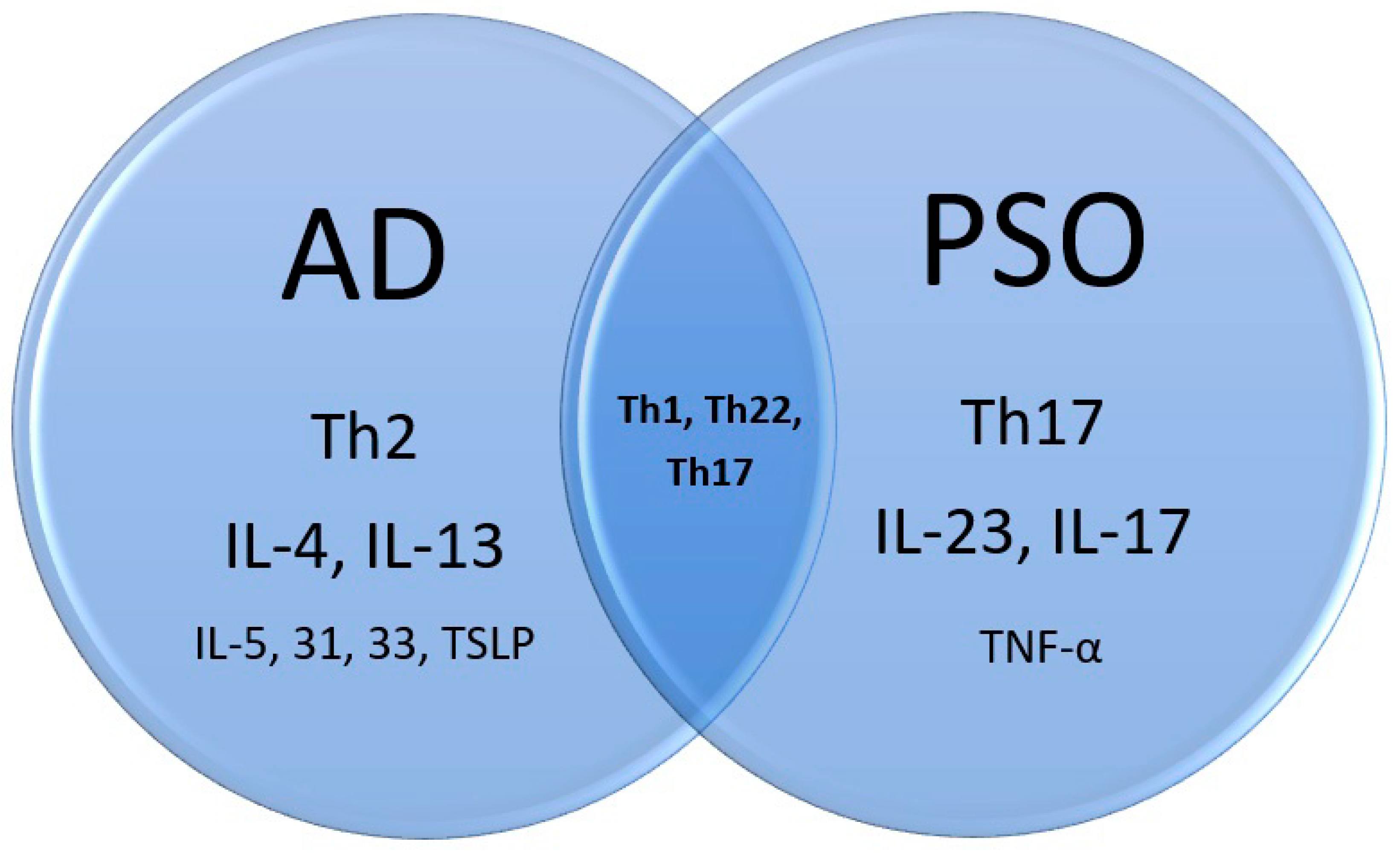
Figure 2. Immunopathogenesis of atopic dermatitis, psoriasis, and the overlap.
IgE was often used clinically as a surrogate serum marker for atopic diathesis, including AD. Despite this, total IgE levels were significantly higher in psoriatic patients than in healthy controls (median IgE 425 IU/mL versus 54.5 IU/mL, p < 0.05) [28][29]. Additionally, patients with a longer period of psoriatic skin lesions had a statistically significant elevation of total IgE levels too [28]. Regarding the mite test measured by the prick test, subjects in PSO and asthmatic groups showed statistically significant positive rates compared to individuals in the healthy control group [29].
In vitro, the scratch injury from both diseases induces CCL20 production by keratinocytes. CCL20 then chemoattracts IL17-producing immune cells. This is another explanation for why the IL-17 amount is also increased in AD [30]. However, pruritus signaling is quite different in AD and PSO. Substance P, IL-2, calcitonin gene-related peptide (CGRP), OPRM, and OPRK are involved in psoriasis-related itch, while thymic stromal lymphopoietin (TSLP), CGRP, IL-4, IL-13, and IL-31 are associated with AD pruritus. Psoriasis itch is mainly induced by transient receptor potential vanilloid 1 (TRPV1) channel, but AD itch is mainly through transient receptor potential ankyrin 1 (TRPA1) [31][32][33].
4. Histopathological Findings
Histopathologically, PSO is characterized by sparse superficial perivascular lymphocytic infiltrates and the extension of lymphocytes into the epidermis in the early phase. It is followed by retention of nuclei (parakeratosis) and mounds of neutrophils (Munro’s microabscesses) in the stratum corneum, elongation of epidermal rete ridges with characteristic bulbous enlargement of their tips or clubbing, i.e., psoriasiform hyperplasia and tortuous vascular ectasias in close proximity to the basal layer [34][35]. In contrast, the histopathological findings of AD are much less characteristic. Intercellular edema within the epidermis, namely spongiosis, is the hallmark of all dermatitis, including AD. The degree of spongiosis depends on the stage of lesions, with more vesiculation in the acute phase and irregular epidermal hyperplasia in the chronic phase (Figure 3).
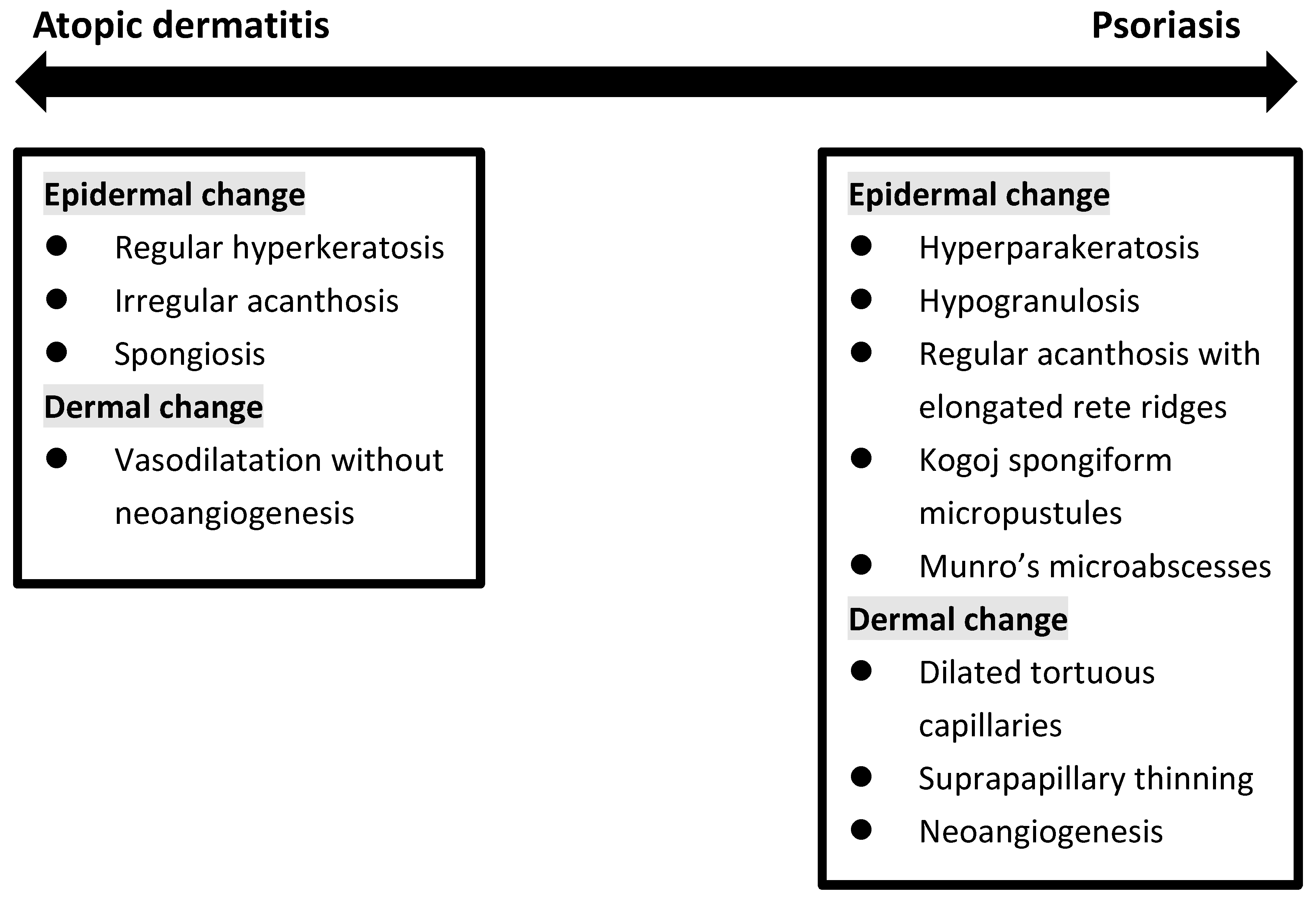
Figure 3. Histopathologic change in between atopic dermatitis and psoriasis.
The diagnosis of inflammatory skin diseases is heavily dependent on clinical signs [36]. However, clinicians sometimes face a dilemma when there are characteristics in between AD and PSO. It is especially problematic when irritation or partial treatment is accompanied by lesions on volar skin and in patients with erythroderma [37]. Pathology is hence the next step to make a further distinction. In actuality, atypical histopathologic features of PSO are noted with high probability because dermatologists rarely perform biopsies on skin lesions that show typical clinical features of PSO for diagnostic purposes. AD, especially in its chronic form and impetiginization, can share many similar histopathologic features with PSO.
Eosinophilic leukocytes were deemed absent in PSO [38], but are regularly observed in AD. In fact, in a case series of 51 clinically confirmed cases of PSO, spongiosis, compact orthokeratosis, dermal plasma cells, and dermal eosinophils were seen in 76%, 37%, 21%, and 49%, respectively. Spongiosis was 100% present in guttate PSO, and eosinophils were identified in 80% of inverse PSO. In palmoplantar PSO, dermal plasma cells were observed in 50% of patients [39]. In another two studies, eosinophils were seen in 46% [40] and 18% [41] of biopsy specimens of PSO. It is not uncommon to see a pathologic diagnosis of psoriasiform spongiotic dermatitis or spongiotic psoriasiform dermatitis, which turned out to be PSO or AD after follow-up.
Among patients with hand eczema, around one-third of moderate-to-severe hand diseases had a history of AD [42]. Non-pustular palmoplantar PSO has considerable clinicopathologic overlaps with hand eczema. In a cohort of 132 patients having palmar inflammation, a mixed histology of eczema and PSO was given by pathologists in 77 patients [43]. For palmoplantar lesions, although findings of psoriasiform hyperplasia, parakeratosis, hypogranulosis, presence of Munro’s microabscesses, and appearance of tortuous and ectatic capillaries in the papillary dermis were more frequently seen in palmoplantar PSO compared with eczematous dermatitis, none of these features were statistically significant. Conversely, spongiotic vesicles were noted in a high proportion of the patients with PSO (76.5%) [44]. A retrospective study revealed similar results: it failed to attain the histopathologic distinction between palmar PSO and hyperkeratotic hand eczema [45]. In one immunohistochemical analysis, hyperkeratotic hand eczema was found to share pathogenesis with palmar PSO, based on the elevated level of β-defensin 2 in the stratum corneum layer and IL-36γ in the stratum granulosum layer in both diseases [46].
5. Shared Comorbidities Focusing on Autoimmune Diseases
A meta-analysis demonstrated that multiple autoimmune diseases had a varying extent of association with AD. This included alopecia areata, vitiligo, celiac disease, ulcerative colitis, Crohn′s disease, rheumatoid arthritis, and systematic lupus erythematosus [47]. Genetic cause has been suggested because there is a greater risk of alopecia areata in patients with filaggrin gene mutation [48]. AD shared 39 genetic loci with inflammatory bowel diseases, which also implied a genetic linkage [48].
In PSO, the prevalence of alopecia areata, vitiligo, rheumatoid arthritis, systemic lupus erythematosus, bullous pemphigoid, and pemphigus were increased (Table 1) [49].
Table 1. Comorbidities of autoimmune diseases between atopic dermatitis and psoriasis.
|
Comorbidities |
Atopic Dermatitis |
Psoriasis |
|
|---|---|---|---|
|
Gastroenterology |
Ulcerative colitis |
V |
Inconsistent data |
|
Crohn’s disease |
V |
V |
|
|
Celiac disease |
V |
V |
|
|
Dermatology |
Vitiligo |
V |
V |
|
Alopecia areata |
V |
V |
|
|
Atopy |
Allergic rhinitis |
V |
V |
|
Asthma |
V |
V |
|
|
Musculoskeletal disease |
Systemic lupus erythematosus |
V |
V |
|
Rheumatoid arthritis |
V |
V |
|
|
Autoimmune bullous disease |
Bullous pemphigoid |
V |
V |
|
Pemphigus |
Unknown |
V |
|
6. Phenotypes of Overlapping Psoriasis and Atopic Dermatitis
Although the diagnosis of typical PSO and AD is usually straightforward, it may be more challenging in the pediatric group or in special locations. In a study of pediatric PSO and AD, only 10% of children with PSO were diagnosed correctly, and 79.9% of patients with PSO were diagnosed as AD by the referring doctors [50]. Lack of experience might be one reason; lack of typical lesions would be another reason. Even dermatologists may sometimes find it difficult to make a clear distinction in 20% of cases that showed a combination of both disease features, so-called psoriasis eczema (PsEma) [51]. Overlapping diseases can be diagnosed concurrently or consecutively. In order to specify various conditions, they were subdivided into five subtypes according to the clinical manifestations, disease course, and transformation induced by medications (Figure 4):
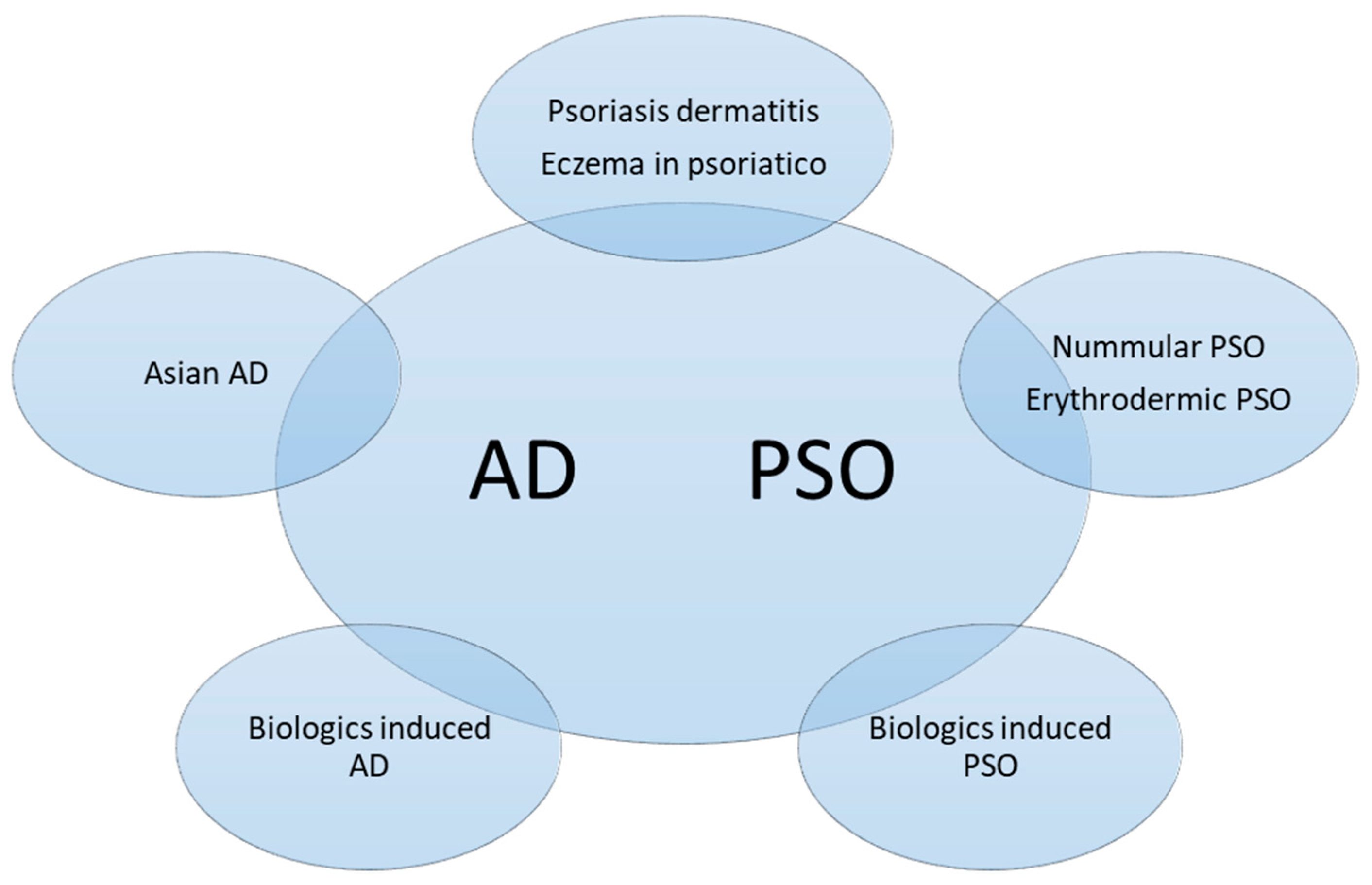
Figure 4. Subtypes of overlapping psoriasis (PSO) and atopic dermatitis (AD). The phenotypes could be classified into concomitant PSO and AD, mainly PSO lesions with AD features or vice versa, or disease transformation as a result of biologics treatment.
- PSO with AD features (Nummular PSO, erythrodermic PSO)
- AD with PSO features (Asian AD)
- Coexisting AD and PSO (Psoriasis dermatitis, PSO-Eczema, PsEma, eczema in psoriatico)
- Development of AD-Like dermatitis during PSO or AD treatment (TNFai, IL-12/23i, IL-17i, IL-23i, IL-4/13i)
- Development of PSO during AD treatment (Dupilumab)
References
- Schäbitz, A.; Eyerich, K.; Garzorz-Stark, N. So close, and yet so far away: The dichotomy of the specific immune response and inflammation in psoriasis and atopic dermatitis. J. Intern. Med. 2021, 290, 27–39.
- Griffiths, C.E.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N. Psoriasis. Lancet 2021, 397, 1301–1315.
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int J. Mol. Sci. 2019, 20, 1475.
- Ständer, S. Atopic Dermatitis. N. Engl. J. Med. 2021, 384, 1136–1143.
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360.
- Moldovan, L.I.; Tsoi, L.C.; Ranjitha, U.; Hager, H.; Weidinger, S.; Gudjonsson, J.E.; Kjems, J.; Kristensen, L.S. Characterization of circular RNA transcriptomes in psoriasis and atopic dermatitis reveals disease-specific expression profiles. Exp. Dermatol. 2021, 30, 1187–1196.
- He, H.; Bissonnette, R.; Wu, J.; Diaz, A.; Proulx, E.S.-C.; Maari, C.; Jack, C.; Louis, M.; Estrada, Y.; Krueger, J.G.; et al. Tape strips detect distinct immune and barrier profiles in atopic dermatitis and psoriasis. J. Allergy Clin. Immunol. 2021, 147, 199–212.
- Henseler, T.; Christophers, E. Disease concomitance in psoriasis. J. Am. Acad. Dermatol. 1995, 32, 982–986.
- Dai, Y.X.; Tai, Y.H.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between psoriasis and atopic dermatitis: A nationwide population-based cohort study. Dermatology 2021, 237, 521–527.
- Barry, K.; Zancanaro, P.; Casseres, R.; Abdat, R.; Dumont, N.; Rosmarin, D. Concomitant atopic dermatitis and psoriasis—A retrospective review. Rev. J. Dermatol. Treat. 2021, 32, 716–720.
- Williams, H.C.; Strachanh, D.P. Psoriasis and Eczema Are Not Mutually Exclusive Diseases. Dermatology 1994, 189, 238–240.
- Beer, W.E.; Smith, A.E.; Kassab, J.Y.; Smith, P.H.; Payne, C.M.R. Concomitance of psoriasis and atopic dermatitis. Dermatology 1992, 184, 265–270.
- Kirsten, N.; Mohr, N.; Maul, J.T.; Augustin, M. Incidence of atopic conditions in people with psoriasis: A population-based analysis. Eur. J. Dermatol. 2021, 31, 60–64.
- Baurecht, H.; Hotze, M.; Brand, S.; Büning, C.; Cormican, P.; Corvin, A.; Ellinghaus, D.; Ellinghaus, E.; Esparza-Gordillo, J.; Fölster-Holst, R.; et al. Genome-wide comparative analysis of atopic dermatitis and psoriasis gives insight into opposing genetic mechanisms. Am. J. Hum. Genet. 2015, 96, 104–120.
- Hüffmeier, U.; Traupe, H.; Oji, V.; Lascorz, J.; Sta¨nder, M.; Lohmann, J.; Wendler, J.; Burkhardt, H.; Reis, A. Loss-of-function variants of the filaggrin gene are not major susceptibility factors for psoriasis vulgaris or psoriatic arthritis in German patients. J. Investig. Dermatol. 2007, 127, 1367–1370.
- Zhao, Y.; Terron-Kwiatkowski, A.; Liao, H.; Lee, S.P.; Allen, M.H.; Hull, P.R.; Campbell, L.E.; Trembath, R.C.; Capon, F.; Griffiths, C.E.; et al. Filaggrin null alleles are not associated with psoriasis. J. Investig. Dermatol. 2007, 127, 1878–1882.
- Bergboer, J.G.; Zeeuwen, P.L.; Irvine, A.D.; Weidinger, S.; Giardina, E.; Novelli, G.; Heijer, M.D.; Rodriguez, E.; Illig, T.; Riveira-Munoz, E.; et al. Deletion of Late Cornified Envelope 3B and 3C genes is not associated with atopic dermatitis. J. Investig. Dermatol. 2010, 130, 2057–2061.
- Chang, Y.C.; Wu, W.M.; Chen, C.H.; Hu, C.F.; Hsu, L.A. Association between P478S polymorphism of the filaggrin gene and risk of psoriasis in a Chinese population in Taiwan. Arch. Dermatol. Res. 2008, 300, 133–137.
- Hu, Z.; Xiong, Z.; Xu, X.; Li, F.; Lu, L.; Li, W.; Su, J.; Liu, Y.; Liu, D.; Xie, Z.; et al. Loss-of-function mutations in filaggrin gene associate with psoriasis vulgaris in Chinese population. Hum. Genet. 2012, 131, 1269–1274.
- Tsoi, L.C.; Rodriguez, E.; Degenhardt, F.; Baurecht, H.; Wehkamp, U.; Volks, N.; Szymczak, S.; Swindell, W.R.; Sarkar, M.K.; Raja, K.; et al. Atopic dermatitis is an IL-13-dominant disease with greater molecular heterogeneity compared to psoriasis. J. Investig. Dermatol. 2019, 139, 1480–1489.
- Wongpiyabovorn, J.; Suto, H.; Ushio, H.; Izuhara, K.; Mitsuishi, K.; Ikeda, S.; Nakao, A.; Okumura, K.; Ogawa, H. Up-regulation of interleukin-13 receptor α1 on human keratinocytes in the skin of psoriasis and atopic dermatitis. J. Dermatol. Sci. 2003, 33, 31–40.
- Bowes, J.; Eyre, S.; Flynn, E.; Ho, P.; Salah, S.; Warren, R.B.; Marzo-Ortega, H.; Coates, L.; McManus, R.; Ryan, A.W.; et al. Evidence to support IL-13 as a risk locus for psoriatic arthritis but not psoriasis vulgaris. Ann. Rheum. Dis. 2011, 70, 1016–1019.
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; Strong, C.D.G.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264.
- Guttman-Yassky, E.; Krueger, J.G. Atopic dermatitis and psoriasis: Two different immune diseases or one spectrum? Curr. Opin. Immunol. 2017, 48, 68–73.
- Bolognia, J.L.; Schaffer, J.V.; Cerroni, L. Psoriasis. In Dermatology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2018; p. 140.
- Tsai, Y.C.; Tsai, T.F. Anti-interleukin and interleukin therapies for psoriasis: Current evidence and clinical usefulness. Adv. Musculoskelet Dis. 2017, 9, 277–294.
- Guttman-Yassky, E.; Brunner, P.M.; Neumann, A.U.; Khattri, S.; Pavel, A.B.; Malik, K.; Singer, G.K.; Baum, D.; Gilleaudeau, P.; Sullivan-Whalen, M.; et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J. Am. Acad. Dermatol. 2018, 78, 872–881.
- Kasumagic-Halilovic, E. Total Serum Immunoglobulin E Levels in Patients with Psoriasis. Mater. Sociomed. 2020, 32, 105–107.
- Ünal, E.S.; Gül, Ü.; Dursun, A.B.; Erkekol, F.Ö. Prediction of atopy via total immunoglobulin E levels and skin prick tests in patients with psoriasis. Turk. J. Med. Sci. 2017, 47, 577–582.
- Furue, K.; Ulzii, D.; Tanaka, Y.; Ito, T.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Pathogenic implication of epidermal scratch injury in psoriasis and atopic dermatitis. J. Dermatol. 2020, 47, 979–988.
- Kahremany, S.; Hofmann, L.; Harari, M.; Gruzman, A.; Cohen, G. Pruritus in psoriasis and atopic dermatitis: Current treatments and new perspectives. Pharm. Rep. 2021, 73, 443–453.
- Komiya, E.; Tominaga, M.; Kamata, Y.; Suga, Y.; Takamori, K. Molecular and cellular mechanisms of itch in psoriasis. Int. J. Mol. Sci. 2020, 21, 8406.
- Xie, B.; Li, X.Y. Inflammatory mediators causing cutaneous chronic itch in some diseases via transient receptor potential channel subfamily V member 1 and subfamily A member 1. J. Dermatol. 2019, 46, 177–185.
- Ragaz, A.; Ackerman, A.B. Evolution, maturation, and regression of lesions of psoriasis. New observations and correlation of clinical and histologic findings. Am. J. Dermatopathol. 1979, 1, 199–214.
- Murphy, M.; Kerr, P.; Grant-Kels, J.M. The histopathologic spectrum of psoriasis. Clin. Dermatol. 2007, 25, 524–528.
- Aslan, C.; Göktay, F.; Mansur, A.T.; Aydıngöz, İ.E.; Güneş, P.; Ekmekçi, T.R. Clinicopathological consistency in skin disorders: A retrospective study of 3949 pathological reports. J. Am. Acad. Dermatol. 2012, 66, 393–400.
- Ibad, S.; Heibel, H.D.; Cockerell, C.J. Specificity of the Histopathologic Diagnosis of Psoriasis. Am. J. Dermatopathol. 2021, 43, 678.
- Helwig, E.B. Pathology of psoriasis. Ann. N. Y. Acad. Sci. 1958, 73, 924–935.
- Chau, T.; Parsi, K.K.; Ogawa, T.; Kiuru, M.; Konia, T.; Li, C.S.; Fung, M.A. Psoriasis or not? Review of 51 clinically confirmed cases reveals an expanded histopathologic spectrum of psoriasis. J. Cutan. Pathol. 2017, 44, 1018–1026.
- Penn, L.; Brinster, N.K. Eosinophils among the histological features of psoriasis. Am. J. Dermatopathol. 2019, 41, 347–349.
- Rosa, G.; Fernandez, A.P.; Schneider, S.; Billings, S.D. Eosinophils are rare in biopsy specimens of psoriasis vulgaris. J. Cutan. Pathol. 2017, 44, 1027–1032.
- Quaade, A.S.; Simonsen, A.B.; Halling, A.S.; Thyssen, J.P.; Johansen, J.D. Prevalence, incidence, and severity of hand eczema in the general population–A systematic review and meta-analysis. Contact Dermat. 2021, 84, 361–374.
- Kolesnik, M.; Franke, I.; Lux, A.; Quist, S.R.; Gollnick, H.P. Eczema in Psoriatico: An Important Differential Diagnosis Between Chronic Allergic Contact Dermatitis and Psoriasis in Palmoplantar Localization. Acta Derm. Venereol. 2018, 98, 50–58.
- Aydin, O.; Engin, B.; Oğuz, O.; İlvan, Ş.; Demirkesen, C. Non-pustular palmoplantar psoriasis: Is histologic differentiation from eczematous dermatitis possible? J. Cutan. Pathol. 2008, 35, 169–173.
- Park, J.Y.; Cho, E.B.; Park, E.J.; Park, H.R.; Kim, K.H.; Kim, K.J. The histopathological differentiation between palmar psoriasis and hand eczema: A retrospective review of 96 cases. J. Am. Acad. Dermatol. 2017, 77, 130–135.
- An, M.K.; Yoon, J.H.; Park, E.J.; Park, H.R.; Kim, K.J.; Kim, K.H. Differential histopathological and immunohistochemical findings between palmar psoriasis and chronic hand eczema. Eur. J. Dermatol. 2020, 30, 710–715.
- Lu, Z.; Zeng, N.; Cheng, Y.; Chen, Y.; Li, Y.; Lu, Q.; Xia, Q.; Luo, D. Atopic dermatitis and risk of autoimmune diseases: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 96.
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major comorbidities of atopic dermatitis: Beyond allergic disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838.
- Tsai, T.F.; Wang, T.S.; Hung, S.T.; Phiona, I.; Tsai, C.; Schenkel, B.; Zhang, M.; Tang, C.H. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J. Derm. Sci. 2011, 63, 40–46.
- Kapila, S.; Hong, E.; Fischer, G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas. J. Derm. 2012, 53, 98–105.
- William, A.; Clay, C.; Lisa, C.S.; Adrian, M.G.; Torsten, E.; Alan, M. PsEma—A hitherto unnamed dermatologic entity with clinical features of both psoriasis and eczema. SKINmed Dermatol. Clin. 2005, 4, 275–281.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
2 times
(View History)
Update Date:
19 May 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




