
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Osman Yusifu Yansaneh | -- | 3810 | 2022-04-23 23:13:40 | | | |
| 2 | Jason Zhu | -38 word(s) | 3772 | 2022-04-24 03:22:34 | | | | |
| 3 | Jason Zhu | -39 word(s) | 3733 | 2022-04-24 03:23:25 | | | | |
| 4 | Jason Zhu | Meta information modification | 3733 | 2022-04-24 03:44:50 | | |
Video Upload Options
With the increase in demand for plastic use, waste plastic (WP) management remains a challenge in the contemporary world due to the lack of sustainable efforts to tackle it. The increment in WPs is proportional to man’s demand and use of plastics, and these come along with environmental challenges. This increase in WPs, and the resulting environmental consequences are mainly due to the characteristic biodegradation properties of plastics. Landfilling, pollution, groundwater contamination, incineration, and blockage of drainages are common environmental challenges associated with WPs. The bulk of these WPs constitutes polyethene (PE), polyethene terephthalate (PET) and polystyrene (PS). Pyrolysis is an eco-friendly thermo-chemical waste plastic treatment solution for valuable product recovery, preferred over landfilling and incineration solutions.
1. Introduction
2. Catalytic Pyrolysis
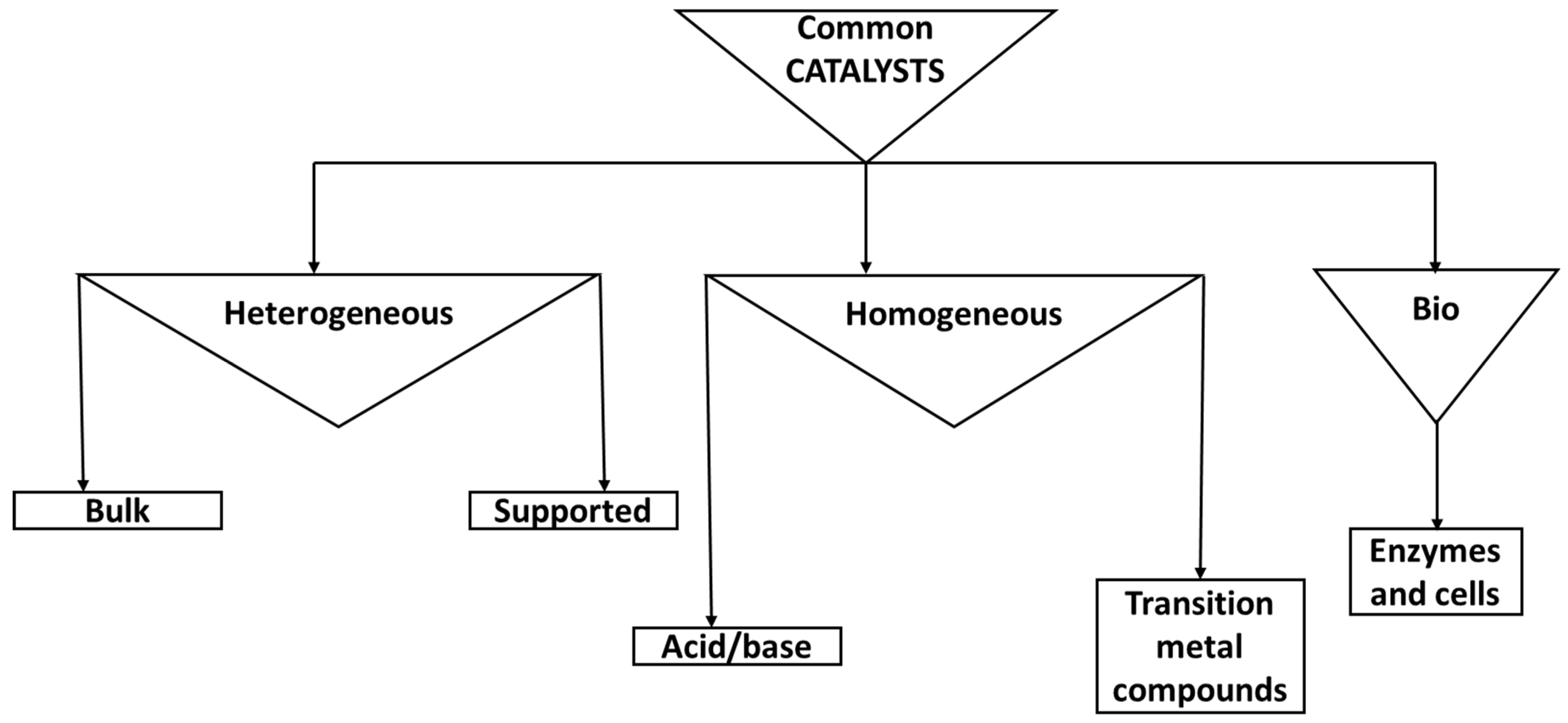
Figure 1. Common catalyst types- with emphasis on heterogeneous and homogeneous catalyses.
2.1. Heterogeneous Catalysts and Pyrolysis
2.2. Homogeneous Catalysts and Pyrolysis
2.3. Biocatalysts and their Reactions
3. Future
Better output with pyrolytic catalysis, an optimal process to its thermal counterpart, is feasible for waste plastic management and pyrolytic product upgrade. The use of catalysts with waste plastic pyrolysis is investigated as more impactful than ordinary thermal cracking. Significant information is shared regarding the potential for converting waste plastics, mixed waste plastics and a combination of waste plastics with other materials such as biomass into fuel fractions and other valuable products. The prominence of heterogeneous catalysis over homogeneous catalysis is widely discussed and their dominance in the economic world. The significance of other catalysts is also investigated. The future of this field is potentially informative.
References
- Pyrolysisadvocacy, (n.d). Pyrolysis Explained. Available online: https://www.pyrolysisadvocacy.com/pyrolysis-explained (accessed on 7 January 2020).
- Demirbas, A. Pyrolysis of municipal plastic waste for recovery of gasoline-range hydrocarbons. J. Anal. Appl. Pyrolysis 2004, 72, 97–102.
- Zafar, S. Pyrolysis of Municipal Wastes. BioEnergy Consult Powering a Greener Future. 29 July 2020. Available online: https://www.bioenergyconsult.com/pyrolysis-of-municipal-waste/ (accessed on 27 November 2021).
- Britannica. “Catalyst”. Chemistry. 2019. Available online: https://www.britannica.com/science/catalyst (accessed on 9 January 2020).
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. A review recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sust. Energ. Rev. 2011, 15, 4171–4186.
- Abnisa, F.; Daud, W.M.A.W. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85.
- Pyrolab (n.d). Sequential Pyrolysis. Available online: https://www.pyrolab.com/pyrolysis-methods/sequential-pyrolysis (accessed on 16 July 2021).
- Syamsiro, M.; Saptoadi, H.; Norsujianto, T.; Noviasri, P.; Cheng, S.; Alimuddin, Z.; Yoshikawaa, K. Fuel Oil Production from Municipal Plastic Wastes in Sequential Pyrolysis and Catalytic Reforming Reactors. 2014. Available online: https://www.sciencedirect.com/science/article/pii/S1876610214002288 (accessed on 9 September 2021).
- Moses, K. Production and Characterization of Liquid Fuel from Mixed Plastic Wastes Using Catalytic Pyrolysis. Master’s Thesis, Makerere University, Kampala, Uganda, 2014.
- Vogt, P.F.; Gerulis, J.J. Amines, Aromatic. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000.
- Miskolczi, N.; Bartha, L.; Deak, G.; Jover, B.; Kallo, D. Thermal and thermo-catalytic degradation of high-density polyethylene waste. J. Anal. Appl. Pyrolysis 2004, 72, 235–242.
- Lee, K.-H. Pyrolysis of Waste Polystyrene and High-Density Polyethylene. In Material Recycling—Trends and Perspectives; In Tech: London, UK, 2012; ISBN 978-953-51-0327-1.
- Marcilla, A.; GarcÃa-Quesada, J.C.; Sánchez, S.; Ruiz, R. Study of the catalytic pyrolysis behaviour of polyethylene polypropylene mixtures. J. Anal. Appl. Pyrolysis 2005, 74, 38792.
- Miskolczi, N.; Bartha, L.; Deak, G.Y. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts. Polym. Degrad. Stab. 2006, 91, 517–526.
- Buekens, A.G.; Huang, H. Catalytic plastics cracking for recovery of gasoline- range hydrocarbons from municipal plastic wastes. Resour. Conserv. Recy. 1998, 23, 163–181.
- Milne, B.J.; Behie, L.A.; Berruti, F. Recycling of waste plastics by ultrapyrolysis using an internally circulating fluidized bed reactor. J. Anal. Appl. Pyrolysis 1999, 51, 157–166.
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27.
- Adnan, A.; Shah, J.; Jan, M.R. Thermo-catalytic pyrolysis of polystyrene in the presence of zinc bulk catalysts. J. Taiwan Inst. Chem. Eng. 2014, 45, 2494–2500.
- Aguado, J.; Serrano, D.P.; Escola, J.M. Catalytic Upgrading of Plastic Wastes. In Feedstock Recycling and Pyrolysis of Waste Plastics; Scheirs, J., Kaminsky, W., Eds.; John Wiley & Sons, Ltd.: Mostoles, Spain, 2006; pp. 73–110.
- DieselNet. Catalyst Fundamentals. DieselNet. Revision 2000.11a. 2000. Available online: https://dieselnet.com/tech/cat_fund.php#:~:text=It%20is%20important%20to%20recognize,the%20kinetics%20of%20reaching%20equilibrium (accessed on 14 August 2020).
- Farnetti, E.; Monte, R.D.; Kašpar, J. Homogeneous and Heterogeneous Catalysis. In Inorganic and Bio-Inorganic Chemistry—(n.d.); ©Encyclopedia of Life Support Systems (EOLSS); University of Trieste: Trieste, Italy, 2010; Volume II.
- Foist, L. Heterogeneous and Homogeneous Catalysts. Available online: https://study.com/academy/lesson/heterogeneous-homogeneous-catalysts.html (accessed on 5 March 2021).
- Pera, L.M.; Baigori, M.D.; Pandey, A.; Castro, G.R. Biocatalysis. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 391–408.
- Yansaneh, O.Y.; Zein, S.H. Recent Advances on Waste Plastic Thermal Pyrolysis: A Critical Overview. Processes 2022, 10, 332.
- Wang, L.; Luo, G.-H.; Li, Q. Progress of waste plastics pyrolysis. Chem. Ind. Eng. Program 2003, 22, 130–134.
- Salaudeen, S.A.; Arku, P.; Dutta, A. Plastics to Energy; Elsevier: Amsterdam, The Netherlands, 2019.
- Ivanova, S.; Gumerova, E.; Minsker, K.; Zaikov, G.; Berlin, A. Selective catalytic degradation of polyolefins. Prog. Polym. Sci. 1990, 15, 193–215.
- Adnan; Shah, J.; Jan, M.R. Effect of polyethylene terephthalate on the catalytic pyrolysis of polystyrene: Investigation of the liquid products. J. Taiwan Inst. Chem. Eng. 2015, 51, 96–102.
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 production from co-pyrolysis/gasification of waste plastics and biomass under novel catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122947.
- Wu, S.L.; Kuo, J.H.; Wey, M.Y. Thermaldegradation of waste plastics in a two-stage pyrolysis-catalysis reactor overcore-shell type catalyst. J. Anal. Appl. Pyrolysis 2019, 142, 104641.
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhöfer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543.
- Maesen, T. The Zeolite Scene—An Overview, 3rd revised ed.; Introduction to Zeolite Science and Practice; Centi, G., Ed.; Elsevier: Richmond, CA, USA, 2007; Volume 1, pp. 1–12.
- ACS Publications. Catalyst bulk phase may play role in oxidation. Chem. Eng. News 1967, 45, 50–53.
- Barrer, R.M. Synthesis of Zeolites; Elsevier: Portoroz, Slovenia, 1985.
- Faravelli, T.; Bozzano, G.; Scassa, C.; Perego, M.; Fabini, S.; Ranzi, E.; Dente, M. Gas product distribution from polyethylene pyrolysis. J. Anal. Appl. Pyrolysis 1999, 52, 87–103.
- Kim, S. Pyrolysis kinetics of waste PVC pipe. Waste Manag. 2001, 21, 609–616.
- Obeid, F.; Zeaiter, J.; Ala’a HAl-Muhtaseb, A.H.; Bouhadir, K. Thermo-catalytic pyrolysis of waste polyethylene bottles in a packed bed reactor with different bed materials and catalysts. Energy Convers. Manag. 2014, 85, 1–6.
- Lumen Learning, (n.d). Homogeneous Catalysis. Introduction to Chemistry. Available online: https://courses.lumenlearning.com (accessed on 5 March 2021).
- Keane, M.A. Catalytic Processing of Waste Polymer Composites. Management, Recycling and Reuse of Waste Composites; Woodhead Publishing Series in Composites Science and Engineering; Woodhead: Cambridge, UK, 2010; pp. 122–151.
- Wang, Y.-F.; Liang, Y.; Wu, Y.-F.; Yang, J.; Zhang, X.; Cai, D.; Peng, X.; Kurmoo, M.; Zeng, M.-H. In Situ Pyrolysis Tracking and Real-Time Phase Evolution: From a Binary Zinc Cluster to Supercapacitive Porous Carbon. Angew. Chem. Int. Ed. 2020, 59, 13232–13237.
- Liu, J.; Liu, J.; Shi, F.; Hu, S.; Jiang, S.; Liu, S.; Liu, D.; Tian, X. Transition metal and co-pyrolysis. Bio Resources. J. Solid. State Chem. 2019, 275, 8–15.
- Park, S.; Bang, Y.; Han, S.J.; Yoo, J.; Song, J.H.; Song, J.C.; Song, I.K. Hydrogen Production by Steam Reforming of Liquefied Natural Gas (LNG) over Mesoporous Nickel-Iron-Alumina Catalyst. J. Mol. Catal. A Chem. 2015, 410, 74–80.
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterisation. J. Master Process. Technol. 2008, 199, 10–26.
- Kakaei, S.; Khameneh, E.S.; Hosseini, M.H.; Moharreri, M.M. A modified ionic liquid clay to remove heavy metals from water: Investigating its catalytic activity. Int. J. Environ. Sci. Technol. 2020, 17, 2043–2058.
- Casella, L. Comprehensive Inorganic Chemistry II—From Elements to Applications, 2nd ed.; Comprehensive Inorganic Chemistry II. Volume Editor’s Introduction; Elsevier: Amsterdam, The Netherlands, 2013; pp. xxxvii–xxxviii.
- Dhawane, S.H.; Halder, G. Synthesis of Catalyst Support from Waste Biomass for Impregnation of Catalysts in Biofuel Production. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–220.
- Kirimura, K.; Ishii, Y. Enzymatic Kolbe–Schmitt Reaction for the Syntheses of Value-Added Compounds. In Future Directions in Biocatalysis, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 135–147.
- Ali, O.M.; Mamat, R.; Rasul, M.G.; Najafi, G. Potential of Biodiesel as Fuel for Diesel Engine. In Clean Energy for Sustainable Development—Comparisons and Contrasts of New Approaches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 557–590.
- Melgarejo-Torres, R.; Pérez-Vega, S.B.; Rivera-Arredondo, V.M.; Che-Galicia, G. Multiphase bioreactors in the pharmaceutical industry. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2019.
- Uragami, T.; Chakraborty, S.; Piemonte, V.; Paola, L.D. Biocatalytic membrane reactors: Principles, preparation and biotechnological, pharmaceutical and medical applications. In Handbook of Membrane Reactors—Reactor Types and Industrial Applications; Woodhead Publishing Series in Energy: Sawston, UK, 2013; Volume 2, pp. 846–887.
- Muthudineshkumar, R.; Anand, R. Anaerobic digestion of various feedstocks for second-generation biofuel production. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2019.
- Wilding, M.; Micklefield, J. Synthetic Methods VI—Enzymatic and Semi-Enzymatic in Comprehensive Chirality; Elsevier BV: Amsterdam, The Netherlands, 2012.
- Pinheiro, B.B.; dos Santos, K.P.; Rios, N.S.; Macedo, A.C.; dos Santos, J.C.S.; Gonçalves, L.R.B. Enzymatic Reactions and Biocatalytic Processes- in Reference Module in Chemistry. Mol. Sci. Chem. Eng. 2019.




