Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alberto García-Peñas | -- | 1909 | 2022-04-18 18:05:10 | | | |
| 2 | Catherine Yang | Meta information modification | 1909 | 2022-04-19 03:29:01 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
García-Peñas, A.; Sharma, G.; Maliki, S.; , .; Moral Zamorano, M.; Baselga, J.; Stadler, F. Chitosan in Sustainable Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/21898 (accessed on 08 February 2026).
García-Peñas A, Sharma G, Maliki S, , Moral Zamorano M, Baselga J, et al. Chitosan in Sustainable Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/21898. Accessed February 08, 2026.
García-Peñas, Alberto, Gaurav Sharma, Soundouss Maliki, , María Moral Zamorano, Juan Baselga, Florian Stadler. "Chitosan in Sustainable Development" Encyclopedia, https://encyclopedia.pub/entry/21898 (accessed February 08, 2026).
García-Peñas, A., Sharma, G., Maliki, S., , ., Moral Zamorano, M., Baselga, J., & Stadler, F. (2022, April 18). Chitosan in Sustainable Development. In Encyclopedia. https://encyclopedia.pub/entry/21898
García-Peñas, Alberto, et al. "Chitosan in Sustainable Development." Encyclopedia. Web. 18 April, 2022.
Copy Citation
The chitosan shows interesting and unique properties, thus it can be used for different purposes which contributes to the design and development of sustainable novel materials. This helps in promoting sustainability through the use of chitosan and diverse materials based on it.
chitosan
sustainable development
circular economy
1. Chitosan as a Renewable Material
1.1. Chitosan as a Biomaterial
Chitosan is obtained through the deacetylation of chitin, which is one of the most abundant biomaterials after cellulose. This one is a polysaccharide which can be found in crustaceans, insects, or fungi (Table 1) [1]. Chitin is considered a linear long-chain homopolymer which is composed of N-acetyl glucosamine, and can develop three polymorphic forms known as α-, β-, and γ-chitin [2].
Table 1. Some of the main chitin sources and percentages [1].
| Source | Percentage (%) |
|---|---|
| Shrimps | 30–40% |
| Squids | 20–40% |
| Krill | 20–30% |
| Crabs | 15–30% |
| Fungi | 10–25% |
| Insects | 5–25% |
| Oysters | 3–6% |
| Clams | 3–6% |
Commercial chitosan (Figure 1) is composed of D-glucosamine and N-acetyl glucosamine and is produced by the partial deacetylation of chitin. This reaction carries out the change of acetamido groups into amino groups. There are three kinds of this biopolymer depending on its molecular weight: low molecular weight, high molecular weight, and oligochitosans [3].
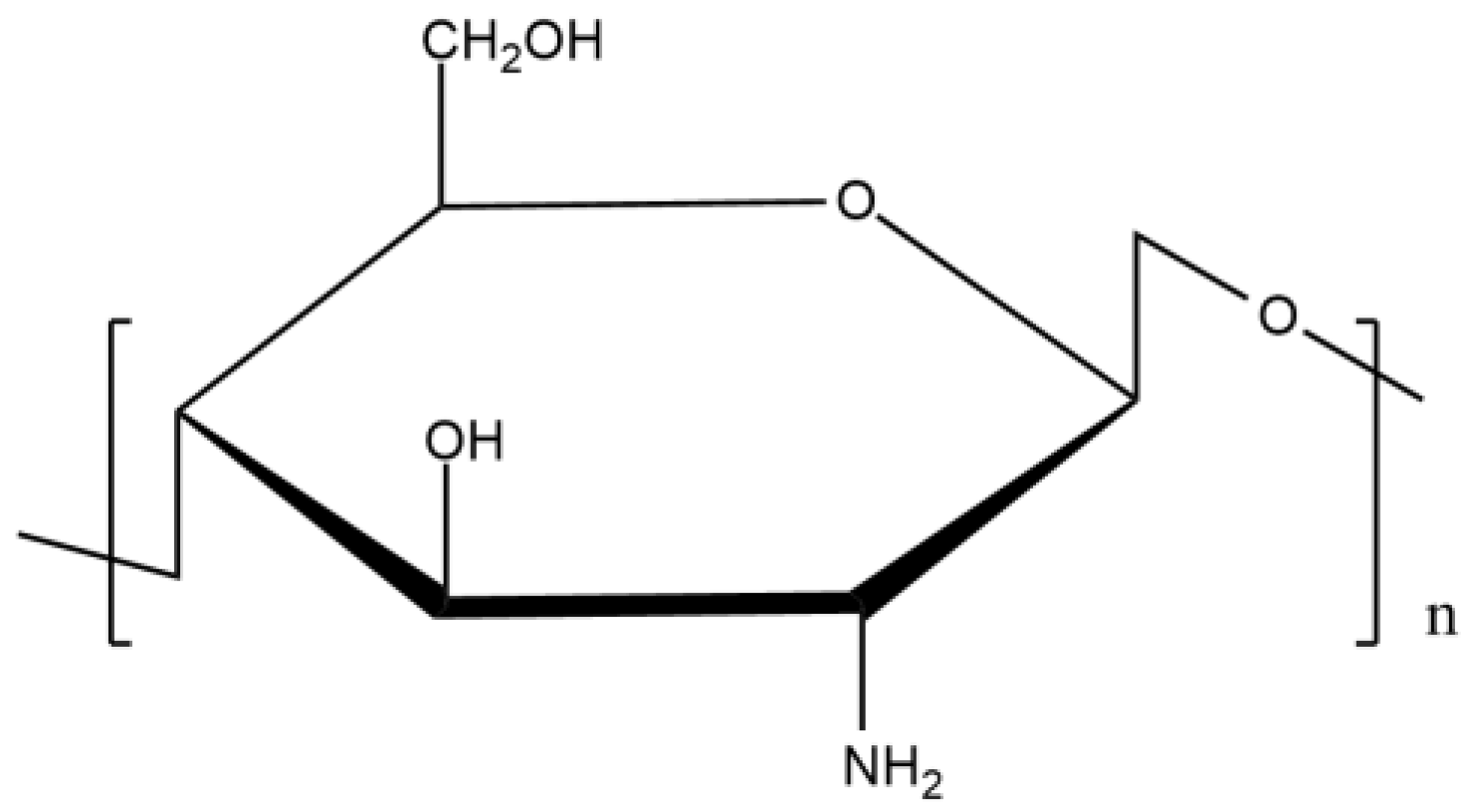
Figure 1. Chemical structure of chitosan.
1.2. General Features and Properties of Chitosan
The main properties which can contribute to a sustainable development that are exhibited by the chitosan are non-toxicity, biodegradability, and biocompatibility. Nevertheless, there are other interesting properties and characteristics which explain its versatility which can be deduced from Table 2.
Table 2. General properties of chitosan [4][5].
| Property | Conditions | Use | References |
|---|---|---|---|
| Solubility | Dilute acids (pH < 6). Insoluble in organic solvents and water | Water treatment | [6][7] |
| Activity | Antibacterial, antifungal mucoadhesive analgesic, and hemostatic properties | [8][9][10] | |
| Degradation | Depends on molecular weight and deacetylation degree | [6][11] | |
| Biocompatibility | Physiological medium | Biomedical applications | [12][13] |
| Chelating properties | Capability to bind and adsorb diverse ions | The removal of heavy metals and dyes from wastewater | [14][15] |
| Biodegradability | Biodegradable to normal body constituents | [13][16][17] | |
| Hemostatic | Stop a hemorrhage | [18][19] | |
| Catalyst | Accelerates the formation of osteoblast | [20] | |
| Fungicide | Stopping the development of fungi | [21][22] | |
| Spermicidal | Reduce the mobility of spermatozoa | [23] | |
| Anticholesteremic | Reducing agent cholesterol | [24][25] | |
| Anticancer | Inhibiting the development of cancer cells | [26] | |
| Conductivity | Ionic conductivity | [27][28] | |
| Flocculating agent | Interactions with negatively charged molecules | Water treatment | [29] |
| Thickener | Increase the viscosity | [30] | |
| Polyelectrolytes | Acidic medium | [31] | |
| Adsorption | Separation and filtration | [32][33][34] | |
| Clarifying agent | Immobilization of enzymes | [35] |
From the presentation of Table 2, it can be deduced that chitosan is a sustainable material as it is biodegradable and non-toxicity [36]. Another important reason for using chitosan is the presence of a large number of hydroxyl and amino groups in its structure which are suitable for chemical modifications [37]. This fact and the wide versatility of chitosan makes this material especially interesting for the preparation of suspensions, composites, functionalized materials, or (nano)hybrids for diverse eco-friendly purposes and applications. The interesting polymorphic behavior exhibited by the chitosan [38], together with the molar mass and degree of deacetylation, mainly defines its mechanical properties. The molar mass will also play an important role for other properties such as degradation degree or antibacterial activity as these are strongly affected by the changes in molar mass.
On the other hand, the degree of deacetylation is associated with the content of acetamide groups of polymeric chains. These groups will strongly affect the final features and properties of the chitosan, in particular its capacity to be biodegradable and its immunological activity. The deacetylation degree is defined between 50 and 99%, its content depends on the preparation methods. The deacetylation degree must be higher than 50% for the chitosan; below that value, it is considered chitin [6]. Some of the most important uses of chitosan are associated with biomedical applications. Nevertheless, new developments related to chitosan focus on agriculture, food packaging, textiles, or environmental applications [39]. The solubility of the chitosan depends on the medium being used to dissolve it; in acid mixtures with water, it is soluble, but it is insoluble in common organic solvents [40][41]. The reason for its solubility can be explained due to the presence of amino groups that transforms chitosan into a base, whose protonation produces a polyelectrolyte [42]. The presence of different functional groups is responsible for the reactivity and the flexibility of this polycationic polymer [43]. Chitosan biofilms show a semi-crystalline behavior, together with high hydrophobicity and little flexibility [44].
1.3. Chitosan as an Ecofriendly Biopolymer and Its Applications
Chitosan is considered a natural biopolymer; it has received remarkable attention from the scientific community due to the fact that it can be easily biodegraded. Its residues are not toxic and can be easily eliminated and biodegraded by nature [12]. One of the most important problems associated with the raw materials is that these are limited, but chitosan is the most abundant biopolymer after cellulose. Furthermore, chitosan exhibits a great biocompatibility, limited by its low solubility which can be solved through chemical modifications and hydrolysis. Chitosan is a bioactive material which can be modulated and used in many applications [45]. Some of these applications are associated with biomedical purposes such as drug delivery systems, scaffolds, or membranes. Nevertheless, there are other important uses such as in the textile industry, wastewater treatments, agriculture, food, packaging, personal care, and biotechnology, among others. The adsorbent properties of chitosan are very useful for removing different heavy metal ions accumulated in water and derived from industrial processes such as Pb2+, Hg2+, and Cu2+, among others [46]. These can be accumulated inside the body and produce numerous diseases [47]. Chitosan can contribute to the agriculture by improving the harvest and productivity, being an ecofriendly material. It is used as a coating for seeds, enhancing the properties of the plants and the obtained products in terms of shelf life. This use as fertilizer is especially useful for plant protection as it can stimulate the plant defense, but it can also act as an antibacterial and antimicrobial agent [48]. Thus, chitosan acts as a plant growth-promoting agent and plant protector [49]. For that reason, it is considered a pesticide by several countries. The antioxidant properties of chitosan, together with its antimicrobial features, are suitable for the production of films for food packaging. The preparation of hybrid materials with chitosan allows modifying the permeability of those films depending on the requirements [50]. The chitosan can also be used as a food additive, dietary fiber, and functional ingredient [51][52].
2. Applications of Chitosan for Sustainable Development
Chitosan can contribute to sustainable development through its applications and uses. This entry tries to expose some of the most important applications related to the contribution of chitosan to a circular economy and sustainability. Figure 2 depicts the diversified application of chitosan.
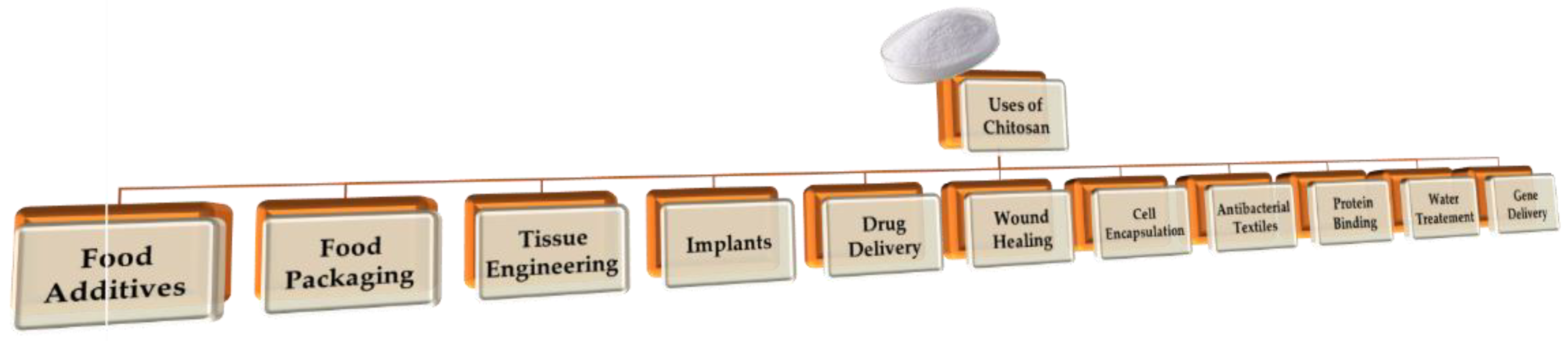
Figure 2. Different uses of chitosan.
2.1. Sustainable Use of Chitosan for Food Packaging and in Agriculture
Many biopolymers are being implemented in different coating materials due to their excellent properties in terms of degradability and compatibility; these biopolymers include gums, starch, proteins cellulose, lipids, and their derivatives [53][54][55][56][57][58][59]. In this sense, chitosan is a promising material for that purpose due to several reasons associated with its biocompatibility and abundance [60][61]. The use of the chitosan in films can also provide other superiorities because of its antibacterial and antioxidant properties [62][63][64][65]. In general, chitosan is used in combination with other polymers due to some of its drawbacks associated with its low mechanical properties. Another important problem associated with chitosan is related to its water sensitivity [66]. The preparation of blends can diminish these problems, thus obtaining films with a wide range of properties. The miscibility problems between the mixtures of polymers can reduce the spectra of possibilities, but in general, the preparation of these films is easy and cheap. The preparation of these systems could be a good alternative regarding traditional films based on oil derivatives [67]. There are other mixtures with synthetic polymer of chitosan that are not included in this entry, as those do not fit the sustainability criteria of the present entry. Numerous composites of chitosan have been fabricated with graphene, carbon nanotubes, activated carbon, and metal nanoparticles [68][69][70][71]. One study suggests that poly(L-lactic acid)-ZnO multilayered with cationic chitosan and anionic β-cyclodextrin can be used as a promising material in applications for the active packaging of food [72]. A novel bilayer food packing film of Ag-Metal−organic framework loaded p-coumaric acid modified chitosan (P-CS/Ag@MOF) or chitosan nanoparticles (P-CSNPs/Ag@MOF) and polyvinyl alcohol/starch (PVA/ST) was fabricated. The bilayer composite film revealed a relatively smooth surface and higher tensile strength (27.67 MPa). The P-CS/Ag@MOF bilayer films displayed better oil resistance and oxidation resistance, and the bilayer film had good UV-blocking properties and transparency [73]. The diverse blend composites of chitosan have been developed with various natural antimicrobial compounds and have been applied for antimicrobial food packaging; such antimicrobial compounds include thyme oil, spirulina, oregano essential oil, nisin, apple peel polyphenols, bamboo vinegar, cinnamon essential oil, custard apple leaves, plum peel extract, etc. [74][75][76][77][78][79][80]. The antibacterial nanofiber films were fabricated using gelatin, chitosan, and 3-phenyllactic acid (PLA) by electrospinning. Under acidic conditions, chitosan and PLA interacted and formed hydrogen bonds, which decreased the crystallinity of the nanofiber films. The nanofiber film had the best thermal stability, water stability, water vapor permeability, and more effective antibacterial effects against Salmonella enterica Enteritidis and Staphylococcus aureus, suggesting that the nanofiber film mat can be used as an active food packaging [81]. Similarly, Wang et al. discussed various chitosan and gelatin edible films, their synthesis strategies including casting, electrospinning, and thermoplastic method, and their properties in their review, thus highlighting importance of chitosan-based food packing films [82]. In Argentina, chitosan is produced from the waste of the shrimp industry; the synthesized chitosan has similar physicochemical properties to those of analytical grade chitosan. The chitosan coatings applied to processed lettuce at harvest increased nutritional quality and reduced microbiological contaminants in minimal processed lettuce [83]. Panda et al. fabricated ferulic acid-modified water-soluble chitosan and poly(γ-glutamic acid) polyelectrolyte multilayers films. These film surfaces possessed a reduced amount of protein adsorption; thus, these can be used as a potential good biomaterial for biomedical purposes to intensify the bio-active surface [84], thus prompting the concept of circularity and sustainability.
The chitosan can act as protector, coating material, stimulator of the growth, nutrient, fertilizer, or pesticide in agriculture. It was also observed that the use of chitosan can increase productivity. Furthermore, the use of chitosan could replace some dangerous chemicals used as compounds of fertilizers in agriculture, protecting soil, aquifers, and ecosystems [85]. It was reported that excellent antimicrobial activity was observed in chitosan against many viruses, bacteria, and fungi. Nevertheless, its activity is higher against fungi than bacteria. In general, the chitosan seems to inactivate the replication of viruses [86]. Moreover, it is considered a potent elicitor which can induce plant defense against diseases [87].
2.2. Sustainable Applications of Chitosan in Purification of Water, Paper-Making, and Green Chemistry
The chitosan is a good flocculant for water treatment, especially indicated for organic matter, suspended solids, and ions (metals). Furthermore, the deposition rate is stimulated when chitosan is used [88]. It is used over oil spills as it can preserve the integrity of the oil mass. Its properties are also indicated for anionic waste where the chitosan can remove the metal ions of the acid solutions. Some of the most attractive features of chitosan regarding other flocculants are associated with its biodegradability and its adsorption and flocculating ability, which show excellent results with oils [12]. Chitosan and its composites demonstrate excellent adsorption properties for diversified environmental contaminates ranging from organic pollutants to metal ions [36][89][90][91][92][93]. The mechanism for the adsorption of toxic pollutants by chitosan and its composites involves various types of interactions such as electrostatic, hydrogen bonding, π-π bonding, etc. The chitosan and its composites had several hydroxyls and amino and carboxylic groups which are very helpful for such interactions, thus making it more adsorbent.
References
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 49–123.
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable Production of Chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Królczyk, G.M., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 45–60.
- Venter, J.P.; Kotze, A.F.; Auzely-Velty, R.; Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 2006, 313, 36–42.
- Dutta, P.K.; Dutta, J.; Tripathi, V.S.; Research, I. Chitin and chitosan: Chemistry, properties and applications. J. Sci. Ind. 2004, 63, 20–31.
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346.
- Tabriz, A.; Ur Rehman Alvi, M.A.; Khan Niazi, M.B.; Batool, M.; Bhatti, M.F.; Khan, A.L.; Khan, A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019, 207, 17–25.
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744.
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984.
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823.
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033.
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083.
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021, 174, 278–288.
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378.
- Kurita, K. Chitin and chitosan: Functional biopolymers from marine crustaceans. Mar. Biotechnol. 2006, 8, 203–226.
- Hoang, H.T.; Jo, S.H.; Phan, Q.T.; Park, H.; Park, S.H.; Oh, C.W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using "click" chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812.
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109.
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147.
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733.
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40.
- Torr, K.M.; Chittenden, C.; Franich, R.A.; Kreber, B. Advances in understanding bioactivity of chitosan and chitosan oligomers against selected wood-inhabiting fungi. Holzforschung 2005, 59, 559–567.
- Pham, D.C.; Nguyen, T.H.; Ngoc, U.T.P.; Le, N.T.T.; Tran, T.V.; Nguyen, D.H. Preparation, Characterization and Antifungal Properties of Chitosan-Silver Nanoparticles Synergize Fungicide Against Pyricularia oryzae. J. Nanosci. Nanotechnol. 2018, 18, 5299–5305.
- Hong, H.-M.; Sim, G.-Y.; Park, S.-M.; Lee, E.-J.; Kim, D.-Y. Ameliorative Effect of Chitosan Complex on Miniature Pig Sperm Cryopreservation. J. Emb. Trans. 2018, 33, 337–342.
- Ahn, S.I.; Cho, S.; Choi, N.J. Effectiveness of Chitosan as a Dietary Supplement in Lowering Cholesterol in Murine Models: A Meta-Analysis. Mar Drugs 2021, 19, 26.
- Lutjohann, D.; Marinova, M.; Wolter, K.; Willinek, W.; Bitterlich, N.; Coenen, M.; Coch, C.; Stellaard, F. Influence of Chitosan Treatment on Surrogate Serum Markers of Cholesterol Metabolism in Obese Subjects. Nutrients 2018, 10, 72.
- Moramkar, N.; Bhatt, P. Insight into chitosan derived nanotherapeutics for anticancer drug delivery and imaging. Eur. Polym. J. 2021, 154, 110540.
- Hadi, J.M.; Aziz, S.B.; Nofal, M.M.; Hussen, S.A.; Hamsan, M.H.; Brza, M.A.; Abdulwahid, R.T.; Kadir, M.F.Z.; Woo, H.J. Electrical, Dielectric Property and Electrochemical Performances of Plasticized Silver Ion-Conducting Chitosan-Based Polymer Nanocomposites. Membranes 2020, 10, 151.
- Vorobiov, V.K.; Smirnov, M.A.; Bobrova, N.V.; Sokolova, M.P. Chitosan-supported deep eutectic solvent as bio-based electrolyte for flexible supercapacitor. Mater. Lett. 2021, 283, 128889.
- Desbrières, J.; Guibal, E. Chitosan for wastewater treatment. Polym. Int. 2018, 67, 7–14.
- Dodero, A.; Brunengo, E.; Alloisio, M.; Sionkowska, A.; Vicini, S.; Castellano, M. Chitosan-based electrospun membranes: Effects of solution viscosity, coagulant and crosslinker. Carbohydr. Polym. 2020, 235, 115976.
- Ferreira, L.M.B.; Dos Santos, A.M.; Boni, F.I.; Dos Santos, K.C.; Robusti, L.M.G.; de Souza, M.P.C.; Ferreira, N.N.; Carvalho, S.G.; Cardoso, V.M.O.; Chorilli, M.; et al. Design of chitosan-based particle systems: A review of the physicochemical foundations for tailored properties. Carbohydr. Polym. 2020, 250, 116968.
- Kordjazi, S.; Kamyab, K.; Hemmatinejad, N. Super-hydrophilic/oleophobic chitosan/acrylamide hydrogel: An efficient water/oil separation filter. Adv. Compos. Hybrid Mater. 2020, 3, 167–176.
- Zhou, G.; Wang, K.P.; Liu, H.W.; Wang, L.; Xiao, X.F.; Dou, D.D.; Fan, Y.B. Three-dimensional polylactic oxide/chitosan sponge bionic filter: Highly efficient adsorption of crystal violet dye. Int. J. Biol. Macromol. 2018, 113, 792–803.
- Hui, M.; Shengyan, P.; Yaqi, H.; Rongxin, Z.; Anatoly, Z.; Wei, C. A highly efficient magnetic chitosan “fluid” adsorbent with a high capacity and fast adsorption kinetics for dyeing wastewater purification. Chem. Eng. J. 2018, 345, 556–565.
- Urrutia, P.; Bernal, C.; Wilson, L.; Illanes, A. Use of chitosan heterofunctionality for enzyme immobilization: Beta-galactosidase immobilization for galacto-oligosaccharide synthesis. Int. J. Biol. Macromol. 2018, 116, 182–193.
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934.
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr. Polym. 2018, 201, 264–279.
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632.
- Brigham, C. Chitin and Chitosan: Sustainable, Medically Relevant Biomaterials. Int. J. Biotech. Well. Indus. 2017, 6, 41–47.
- Lehnert, R.J.; Kandelbauer, A. Comments on “Solubility parameter of chitin and chitosan” Carbohydrate Polymers 36 (1998) 121–127. Carbohydr. Polym. 2017, 175, 601–602.
- Ravindra, R.; Krovvidi, K.R.; Khan, A.A. Solubility parameter of chitin and chitosan. Carbohydr. Polym. 1998, 36, 121–127.
- Pardo-Castaño, C.; Bolaños, G. Solubility of chitosan in aqueous acetic acid and pressurized carbon dioxide-water: Experimental equilibrium and solubilization kinetics. J. Supercrit. Fluids. 2019, 151, 63–74.
- Cunha, R.A.; Soares, T.A.; Rusu, V.H.; Pontes, F.J.; Franca, E.F.; Lins, R.D. The Molecular Structure and Conformational Dynamics of Chitosan Polymers: An Integrated Perspective from Experiments and Computational Simulations. In The Complex World of Polysaccharides; BoD–Books on Demand: Norderstedt, Germany, 2012.
- Uragami, T.T. Material Science of Chitin and Chitosan; Kodansha: Tokyo, Japan, 2006.
- Liu, X.; Wu, Y.; Zhao, X.; Wang, Z. Fabrication and applications of bioactive chitosan-based organic-inorganic hybrid materials: A review. Carbohydr. Polym. 2021, 267, 118179.
- Bi, J.; Huang, X.; Wang, J.; Wang, T.; Wu, H.; Yang, J.; Lu, H.; Hao, H. Oil-phase cyclic magnetic adsorption to synthesize Fe3O4@2-nanotube composites for simultaneous removal of Pb(II) and Rhodamine B. Chem. Eng. J. 2019, 366, 50–61.
- Yan, Y.; Dong, X.; Sun, X.; Sun, X.; Li, J.; Shen, J.; Han, W.; Liu, X.; Wang, L. Conversion of waste FGD gypsum into hydroxyapatite for removal of Pb2+ and Cd2+ from wastewater. J. Colloid Interface Sci. 2014, 429, 68–76.
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819.
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112.
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chapter 15-Chitosan Applications in Food Industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 469–491.
- Vidanarachchi, J.K.; Kim, S.K. Chitin, Chitosan, Oligosaccharides and Their Derivatives. Biological Activities and Applications; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 666.
- Gutiérrez, T.J. Chapter 8: Chitosan Applications for the Food Industry. In Chitosan: Derivatives, Composites and Applications; Ahmed, S., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2017.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Deng, J.; Zhu, E.-Q.; Xu, G.-F.; Naik, N.; Murugadoss, V.; Ma, M.-G.; Guo, Z.; Shi, Z.-J. Overview of renewable polysaccharide-based composites for biodegradable food packaging applications. Green Chem. 2022, 24, 480–492.
- Sharma, G.; Khosla, A.; Kumar, A.; Kaushal, N.; Sharma, S.; Naushad, M.; Vo, D.-V.N.; Iqbal, J.; Stadler, F.J. A comprehensive review on the removal of noxious pollutants using carrageenan based advanced adsorbents. Chemosphere 2022, 289, 133100.
- Sharma, G.; Kumar, A.; Ghfar, A.A.; García-Peñas, A.; Naushad, M.; Stadler, F.J. Fabrication and Characterization of Xanthan Gum-cl-poly(acrylamide-co-alginic acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels 2022, 8, 23.
- Sharma, G.; Kumar, A.; Chauhan, C.; Okram, A.; Sharma, S.; Pathania, D.; Kalia, S. Pectin-crosslinked-guar gum/SPION nanocomposite hydrogel for adsorption of m-cresol and o-chlorophenol. Sustain. Chem. Pharm. 2017, 6, 96–106.
- Elanchezhiyan, S.S.; Preethi, J.; Rathinam, K.; Njaramba, L.K.; Park, C.M. Synthesis of magnetic chitosan biopolymeric spheres and their adsorption performances for PFOA and PFOS from aqueous environment. Carbohydr. Polym. 2021, 267, 118165.
- Sharma, G.; Kumar, A.; Naushad, M.; Thakur, B.; Vo, D.-V.N.; Gao, B.; Al-Kahtani, A.A.; Stadler, F.J. Adsorptional-photocatalytic removal of fast sulphon black dye by using chitin-cl-poly(itaconic acid-co-acrylamide)/zirconium tungstate nanocomposite hydrogel. J. Hazard. Mater. 2021, 416, 125714.
- Leceta, I.; Molinaro, S.; Guerrero, P.; Kerry, J.P.; de la Caba, K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol. Technol. 2015, 100, 142–150.
- Liu, S.; Gao, J.; Zhang, L.; Yang, Y.; Liu, X. Diethylenetriaminepentaacetic acid–thiourea-modified magnetic chitosan for adsorption of hexavalent chromium from aqueous solutions. Carbohydr. Polym. 2021, 274, 118555.
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209.
- Elmehbad, N.Y.; Mohamed, N.A.; Abd El-Ghany, N.A. Evaluation of the antimicrobial and anti-biofilm activity of novel salicylhydrazido chitosan derivatives impregnated with titanium dioxide nanoparticles. Int. J. Biol. Macromol. 2022, 205, 719–730.
- Rodrigues, P.R.; Junior, L.M.; de Souza, W.F.C.; Sato, H.H.; Alves, R.M.V.; Vieira, R.P. O-ATRP synthesized poly(β-pinene) blended with chitosan for antimicrobial and antioxidant bio-based films production. Int. J. Biol. Macromol. 2021, 193, 425–432.
- Nadira, P.P.; Mujeeb, V.M.A.; Rahman, P.M.; Muraleedharan, K. Effects of cashew leaf extract on physicochemical, antioxidant, and antimicrobial properties of N, O–Carboxymethyl chitosan films. Carbohydr. Polym. Technol. Appl. 2022, 3, 100191.
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1819–1841.
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551–100567.
- Panda, P.K.; Dash, P.; Yang, J.-M.; Chang, Y.-H. Development of chitosan, graphene oxide, and cerium oxide composite blended films: Structural, physical, and functional properties. Cellulose 2022, 29, 2399–2411.
- Kim, D.S.; Dhand, V.; Rhee, K.Y.; Park, S.-J. Study on the Effect of Silanization and Improvement in the Tensile Behavior of Graphene-Chitosan-Composite. Polymers 2015, 7, 527–551.
- Sharma, G.; Naushad, M.; Kumar, A.; Kumar, A.; Ahamad, T.; Stadler, F.J. Facile fabrication of chitosan-cl-poly(AA)/ZrPO4 nanocomposite for remediation of rhodamine B and antimicrobial activity. J. King Saud Univ. Sci. 2020, 32, 1359–1365.
- You, J.; Liu, C.; Feng, X.; Lu, B.; Xia, L.; Zhuang, X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption–photocatalytic removal of Congo red. Carbohydr. Polym. 2022, 288, 119332.
- Andrade-Del Olmo, J.; Pérez-Álvarez, L.; Hernáez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)- β-cyclodextrin onto polylactic acid (PLLA). Food Hydrocoll. 2019, 88, 228–236.
- Zhang, M.; Zheng, Y.; Jin, Y.; Wang, D.; Wang, G.; Zhang, X.; Li, Y.; Lee, S. p-coumaric acid modified chitosan/chitosan nanoparticle and polyvinyl alcohol/starch bilayer films for food packing applications. Int. J. Biol. Macromol. 2022, 202, 80–90.
- Zhang, H.; He, P.; Kang, H.; Li, X. Antioxidant and antimicrobial effects of edible coating based on chitosan and bamboo vinegar in ready to cook pork chops. LWT 2018, 93, 470–476.
- Balti, R.; Mansour, M.B.; Sayari, N.; Yacoubi, L.; Rabaoui, L.; Brodu, N.; Massé, A. Development and characterization of bioactive edible films from spider crab (Maja crispata) chitosan incorporated with Spirulina extract. Int. J. Biol. Macromol. 2017, 105, 1464–1472.
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H.; Gardini, F. Chitosan-cinnamon essential oil nano-formulation: Application as a novel additive for controlled release and shelf life extension of beef patties. Int. J. Biol. Macromol. 2017, 102, 19–28.
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y.; Han, X.; Pu, B.; Hu, B.; Deng, W.; et al. Antimicrobial Activities of Nisin, Tea Polyphenols, and Chitosan and their Combinations in Chilled Mutton. J. Food Sci. 2016, 81, M1466–M1471.
- Khanjari, A.; Karabagias, I.K.; Kontominas, M.G. Combined effect of N,O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT Food Sci. Technol. 2013, 53, 94–99.
- Bautista-Baños, S.; Hernández-López, M.; Bosquez-Molina, E.; Wilson, C.L. Effects of chitosan and plant extracts on growth of Colletotrichum gloeosporioides, anthracnose levels and quality of papaya fruit. Crop Prot. 2003, 22, 1087–1092.
- Chamanara, V.; Shabanpour, B.; Gorgin, S.; Khomeiri, M. An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int. J. Biol. Macromol. 2012, 50, 540–544.
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170.
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871.
- Fasciglione, G.; Goñi, M.G.; Yommi, A.K.; Perez-Bravo, J.J.; Ortueta, R.; Scampini, A.; Buffa, L.; Andreu, A.B.; Creus, C.M. Revaluation of waste from fishing industry through generation of chitosan coatings to improve quality and extend shelf-life of minimally processed lettuce. Postharvest Biol. Technol. 2020, 170, 111310.
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H. Preparation and characterization of ferulic acid-modified water soluble chitosan and poly (γ-glutamic acid) polyelectrolyte films through layer-by-layer assembly towards protein adsorption. Int. J. Biol. Macromol. 2021, 171, 457–464.
- Orzali, L.; Corsi, B.; Forni, C.; Riccioni, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. In Biological Activities and Application of Marine Polysaccharides; BoD–Books on Demand: Norderstedt, Germany, 2017.
- Kulikov, S.N.; Chirkov, S.N.; Il’ina, A.V.; Lopatin, S.A.; Varlamov, V.P. Effect of the molecular weight of chitosan on its antiviral activity in plants. Prikl. Biokhim. Mikrobiol. 2006, 42, 224–228.
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49.
- Kangama, A.; Zeng, D.; Tian, X.; Fang, J. Application of Chitosan Composite Flocculant in Tap Water Treatment. J. Chem. 2018, 2018, 2768474.
- Abhinaya, M.; Parthiban, R.; Kumar, P.S.; Vo, D.-V.N. A review on cleaner strategies for extraction of chitosan and its application in toxic pollutant removal. Environ. Res. 2021, 196, 110996.
- Sadiq, A.C.; Olasupo, A.; Ngah, W.S.W.; Rahim, N.Y.; Suah, F.B.M. A decade development in the application of chitosan-based materials for dye adsorption: A short review. Int. J. Biol. Macromol. 2021, 191, 1151–1163.
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889.
- Li, Y.; Liang, Y.-Q.; Mao, X.-M.; Li, H. Efficient removal of Cu(II) from an aqueous solution using a novel chitosan assisted EDTA-intercalated hydrotalcite-like compound composite: Preparation, characterization, and adsorption mechanism. Chem. Eng. J. 2022, 438, 135531.
- Alsamman, M.T.; Sánchez, J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water. Arabian J. Chem. 2021, 14, 103455.
More
Information
Subjects:
Materials Science, Biomaterials
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Environmental Sciences
Revisions:
2 times
(View History)
Update Date:
19 Apr 2022
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




