
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhaowu Ma | -- | 2819 | 2022-04-10 16:36:29 | | | |
| 2 | Jessie Wu | -43 word(s) | 2776 | 2022-04-11 03:06:45 | | | | |
| 3 | Jessie Wu | Meta information modification | 2776 | 2022-04-11 03:07:52 | | | | |
| 4 | Jessie Wu | Meta information modification | 2776 | 2022-04-11 08:47:51 | | | | |
| 5 | Jessie Wu | Meta information modification | 2776 | 2022-04-11 08:49:37 | | |
Video Upload Options
Extracellular vesicles (EVs) act as multifunctional regulators of intercellular communication and are involved in diverse tumor phenotypes, including tumor angiogenesis, which is a highly regulated multi-step process for the formation of new blood vessels that contribute to tumor proliferation. EVs induce malignant transformation of distinct cells by transferring DNAs, proteins, lipids, and RNAs, including noncoding RNAs (ncRNAs). However, the functional relevance of EV-derived ncRNAs in tumor angiogenesis remains to be elucidated.
1. Characteristics of Extracellular Vesicles and ncRNAs
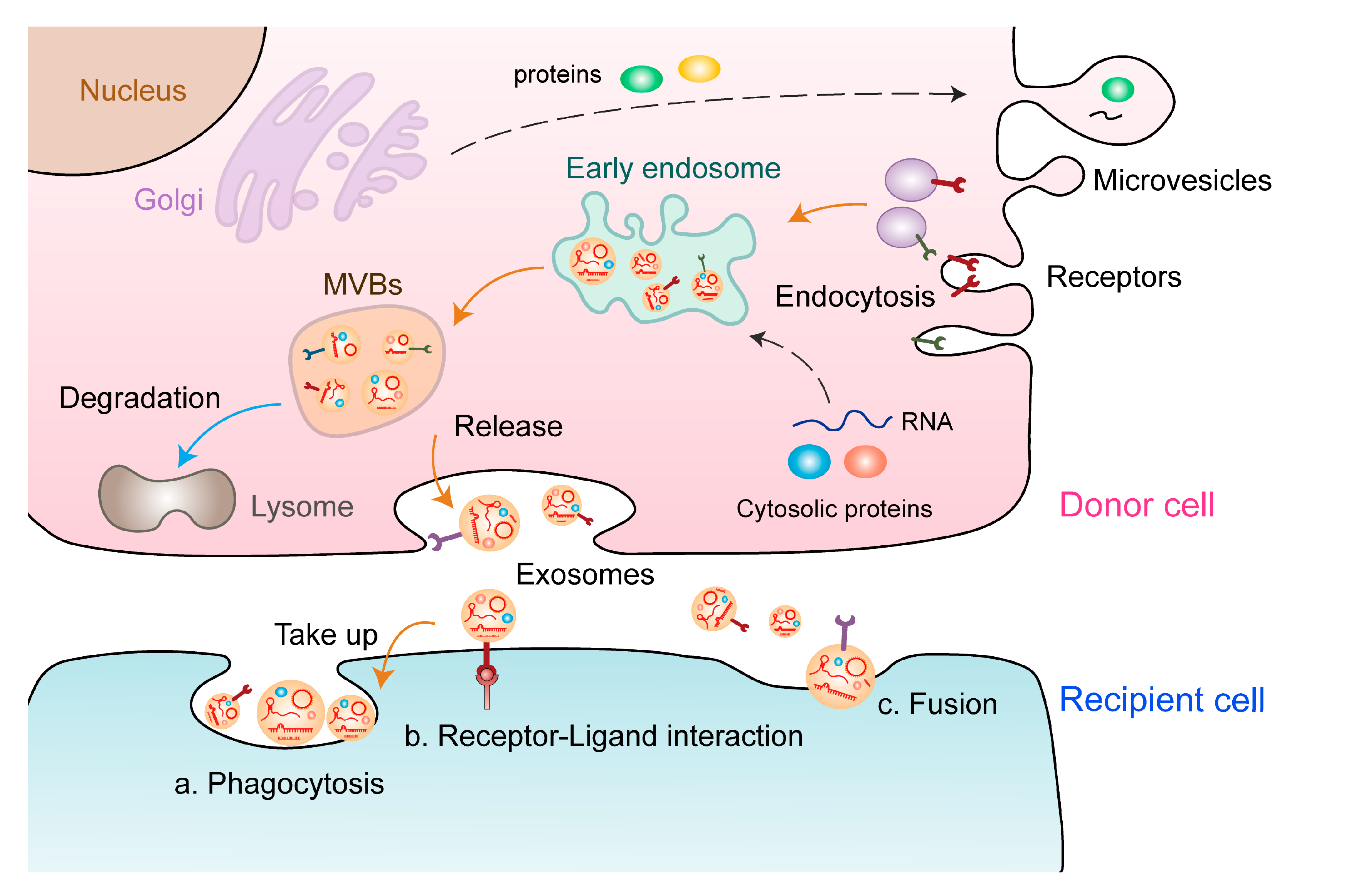
2. EV-Derived ncRNAs: New Players in Tumor Angiogenesis
| EV-Derived ncRNAs | Expression | Source Cell | Function and Mechanism | Tumor Type | Reference |
|---|---|---|---|---|---|
|
miR-155 |
Upregulated |
Tumor cell |
Promotes angiogenesis via the c-MYB/VEGF axis |
Gastric cancer |
[38] |
|
Upregulated |
Tumor cell |
Promotes angiogenesis by inhibiting FOXO3a |
Gastric cancer |
[39] | |
|
miR-130a |
Upregulated |
Tumor cell |
Activates angiogenesis by inhibiting c-MYB |
Gastric cancer |
[40] |
|
miR-135b |
Upregulated |
Tumor cell |
Promotes angiogenesis by inhibiting FOXO1 |
Gastric cancer |
[41] |
|
Upregulated |
Tumor cell |
Regulates the HIF/FIH signalling pathway |
Multiple myeloma |
[42] | |
|
miR-23a |
Upregulated |
Tumor cell |
Inhibits PTEN and activates the AKT pathway |
Gastric cancer |
[43] |
|
Upregulated |
Tumor cell |
Increases angiogenesis by inhibiting ZO-1 |
Lung cancer |
[44] | |
|
miR-200b-3p |
Downregulated |
Tumor cell |
Enhances endothelial ERG expression |
Hepatocellular carcinoma |
[45] |
|
miR-25-3p |
Upregulated |
Tumor cell |
Inhibits KLF2 and KLF4, thereby elevating VEGFR2 expression |
Colorectal cancer |
[46] |
|
miR-1229 |
Upregulated |
Tumor cell |
Inhibits HIPK2, thereby activating the VEGF pathway |
Colorectal cancer |
[47] |
|
miR-183-5p |
Upregulated |
Tumor cell |
Inhibits FOXO1, thereby promoting expression of VEGFA, VEGFAR2, ANG2, PIGF, MMP-2, and MMP-9 |
Colorectal cancer |
[48] |
|
miR-142-3p |
Upregulated |
Tumor cell |
Inhibits TGFβR1 |
Lung adenocarcinoma |
[49] |
|
miR-103a |
Upregulated |
Tumor cell |
Inhibits PTEN, thereby promoting the polarization of M2 macrophages |
Lung cancer |
[50] |
|
miR-126 |
Upregulated |
MSCs |
Upregulates CD34 and CXCR4, thereby promoting expression of VEGF |
Lung cancer |
[51] |
|
miR-141-3p |
Upregulated |
Tumor cell |
Inhibits SOCS5, thereby activating JAK/STAT3 and NF-κB signalling pathways |
Ovarian cancer |
[52] |
|
miR-205 |
Upregulated |
Tumor cell |
Regulates the PTEN/AKT pathway |
Ovarian cancer |
[53] |
|
miR-9 |
Downregulated |
Tumor cell |
Inhibits MDK, thereby regulating the PDK/AKT signalling pathway |
Nasopharyngeal carcinoma |
[54] |
|
Upregulated |
Tumor cell |
Promotes angiogenesis by targeting COL18A1, THBS2, PTCH1, and PHD3 |
Glioma |
[55] | |
|
miR-23a |
Upregulated |
Tumor cell |
Promotes angiogenesis by inhibiting TSGA10 |
Nasopharyngeal carcinoma |
[56] |
|
miR-210 |
Upregulated |
Tumor cell |
Enhances tube formation by inhibiting EFNA3 |
Leukemia |
[57] |
|
Upregulated |
Tumor cell |
Promotes angiogenesis by inhibiting SMAD4 and STAT6 |
Hepatocellular carcinoma |
[58] | |
|
miR-26a |
Upregulated |
Tumor cell |
Inhibits PTEN, thereby activating the PI3K/AKT signalling pathway |
Glioma |
[59] |
|
miR-27a |
Upregulated |
Tumor cell |
Inhibits BTG2, thereby promoting VEGF, VEGFR, MMP-2, and MMP-9 expression |
Pancreatic cancer |
[60] |
|
miR-155-5p /miR-221-5p |
Upregulated |
M2 macrophages |
Promotes angiogenesis by targeting E2F2 |
Pancreatic cancer |
[61] |
|
miR-21-5p |
Upregulated |
Tumor cell |
Promotes angiogenesis by targeting TGFBI and COL4A1 |
Papillary carcinoma |
[62] |
|
miR-100 |
- - |
MSCs |
Regulates the mTOR/HIF-1α signalling axis |
Breast cancer |
[63] |
|
miR-21 |
Upregulated |
Tumor cell |
Inhibits SPRY1, thereby promoting VEGF expression |
Oesophageal squamous cell carcinoma |
[64] |
|
Upregulated |
Tumor cell |
Inhibits PTEN, thereby activating PDK1/AKT signalling |
Hepatocellular carcinoma |
[65] | |
|
miR-181b-5p |
Upregulated |
Tumor cell |
Inhibits PTEN and PHLPP2, thereby activating AKT signalling |
Oesophageal squamous cell carcinoma |
[66] |
|
miR-9 |
Upregulated |
Tumor cell |
Inhibits S1P, thereby promoting VEGF expression |
Medulloblastoma and xenoglioblastoma |
[67] |
|
miR-10a-5p |
Upregulated |
CAFs |
Inhibits TBX5, thereby activating Hedgehog signalling |
Cervical squamous cell carcinoma |
[68] |
|
miR-135b |
Upregulated |
Tumor cell |
Enhances angiogenesis by targeting FIH-1 |
Multiple myeloma |
[42] |
|
miR-130b-3p |
Upregulated |
M2 macrophages |
Regulates the miR-130b-3p/MLL3/GRHL2 signalling cascade |
Gastric cancer |
[69] |
|
lncGAS5 |
Downregulated |
Tumor cell |
Inhibits angiogenesis by regulating the miR-29-3p/PTEN axis |
Lung cancer |
[70] |
|
lnc-CCAT2 |
Upregulated |
Tumor cell |
Promotes VEGFA and TGF-β expression |
Glioma |
[71] |
|
lnc-POU3F3 |
Upregulated |
Tumor cell |
Promotes bFGF, bFGFR, and VEGFA expression |
Glioma |
[72] |
|
lncRNA RAMP2-AS1 |
Upregulated |
Tumor cell |
Promotes angiogenesis through the miR-2355-5p/VEGFR2 axis |
Chondrosarcoma |
[73] |
|
OIP5-AS1 |
Upregulated |
Tumor cell |
Regulates angiogenesis and autophagy through miR-153/ATG5 axis |
Osteosarcoma |
[74] |
|
FAM225A |
Upregulated |
Tumor cell |
Promotes angiogenesis through the miR-206/NETO2/FOXP1 axis |
Oesophageal squamous cell carcinoma |
[36] |
|
UCA1 |
Upregulated |
Tumor cell |
Promotes angiogenesis through the miR-96-5p/AMOTL2 axis |
Pancreatic cancer |
[75] |
|
SNHG11 |
Upregulated |
Tumor cell |
Promotes angiogenesis through the miR-324-3p/VEGFA axis |
Pancreatic cancer |
[76] |
|
SNHG1 |
Upregulated |
Tumor cell |
Promotes angiogenesis by regulating the miR-216b-5p/JAK2 axis |
Breast cancer |
[77] |
|
AC073352.1 |
Upregulated |
Tumor cell |
Binds and stabilizes the YBX1 protein |
Breast cancer |
[78] |
|
MALAT1 |
Upregulated |
Tumor cell |
Facilitates angiogenesis and predicts poor prognosis |
Ovarian cancer |
[79] |
|
TUG1 |
Upregulated |
Tumor cell |
Promotes angiogenesis by inhibiting caspase-3 activity |
Cervical cancer |
[80] |
|
LINC00161 |
Upregulated |
Tumor cell |
Promotes angiogenesis and metastasis by regulating the miR-590-3p/ROCK axis |
Hepatocellular carcinoma |
[81] |
|
H19 |
Upregulated |
Cancer stem cell |
Promotes VEGF production and release in ECs |
Liver cancer |
[82] |
|
circSHKBP1 |
Upregulated |
Tumor cell |
Enhances VEGF mRNA stability by the miR-582-3p/HUR axis |
Gastric cancer |
[37] |
|
circRNA-100,338 |
Upregulated |
Tumor cell |
Facilitates HCC metastasis by enhancing invasiveness and angiogenesis |
Hepatocellular carcinoma |
[83] |
|
circCMTM3 |
Upregulated |
Tumor cell |
Promotes angiogenesis and HCC tumor growth by the miR-3619-5p/SOX9 axis |
Hepatocellular carcinoma |
[84] |
|
circ_0007334 |
Upregulated |
Tumor cell |
Accelerates CRC tumor growth and angiogenesis by the miR-577/KLF12 axis |
Colorectal cancer |
[85] |
|
CircFNDC3B |
Downregulated |
Tumor cell |
Inhibits angiogenesis and CRC progression by the miR-937-5p/TIMP3 axis |
Colorectal cancer |
[86] |
|
circGLIS3 |
Upregulated |
Tumor cell |
Induces endothelial cell angiogenesis by promoting Ezrin T567 phosphorylation |
Glioma |
[87] |
|
piRNA-823 |
Upregulated |
Tumor cell |
Promotes VEGF and IL-6 expression |
Multiple myeloma |
[88] |
3. Potential Clinical Applications of EV-Derived ncRNAs in Cancers
3.1. EV-Derived ncRNAs as Promising Tumor Biomarkers
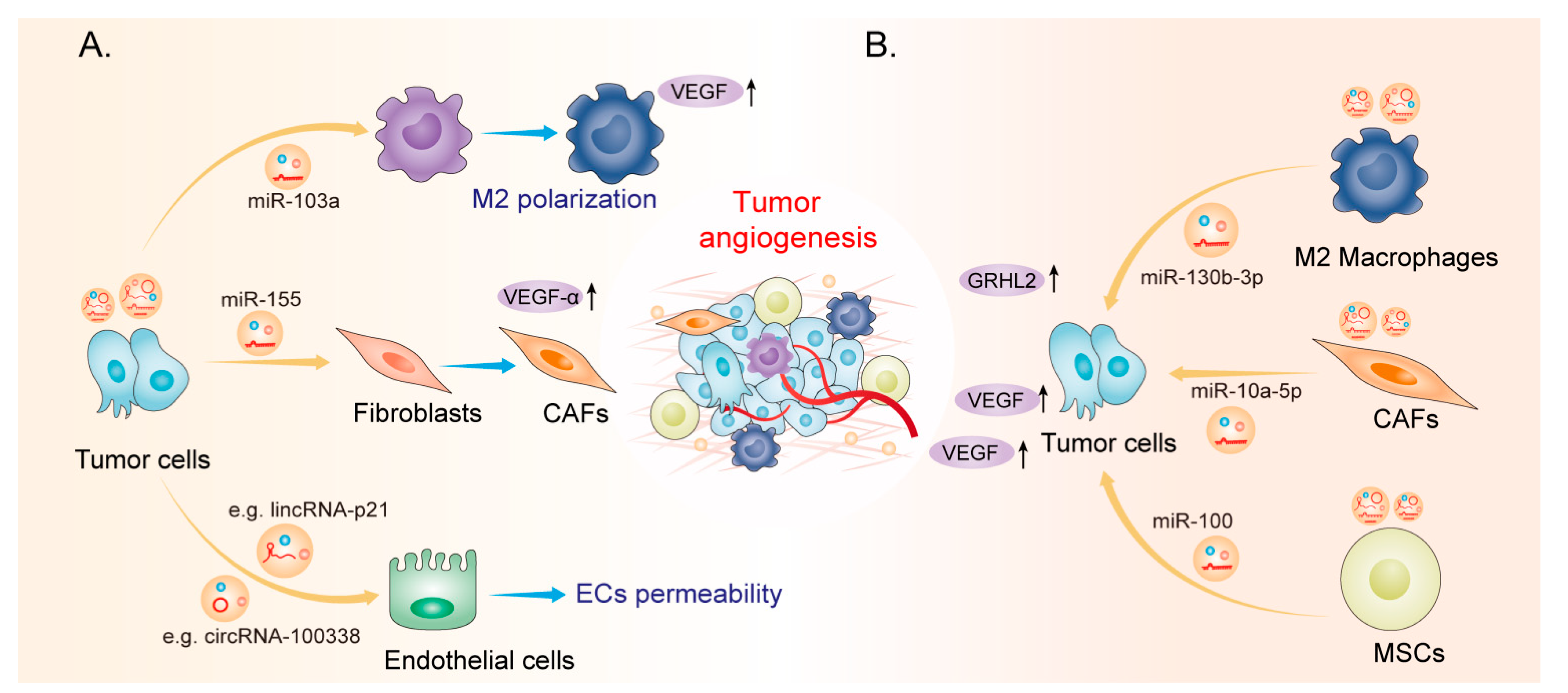
3.2 EV-Derived ncRNAs as Potential Anti-Angiogenic Therapeutic Targets
References
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. 2011, 68, 2667–2688.
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Christ, L.; Raiborg, C.; Wenzel, E.M.; Campsteijn, C.; Stenmark, H. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem. Sci. 2016, 42, 42–56.
- Stuffers, S.; Wegner, C.S.; Stenmark, H.; Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 2009, 10, 925–937.
- Kikuchi, S.; Yoshioka, Y.; Prieto-Vila, M.; Ochiya, T. Involvement of Extracellular Vesicles in Vascular-Related Functions in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 2584.
- Nagano, T.; Fraser, P. No-Nonsense Functions for Long Noncoding RNAs. Cell 2011, 145, 178–181.
- Ingenito, F.; Roscigno, G.; Affinito, A.; Nuzzo, S.; Scognamiglio, I.; Quintavalle, C.; Condorelli, G. The Role of Exo-miRNAs in Cancer: A Focus on Therapeutic and Diagnostic Applications. Int. J. Mol. Sci. 2019, 20, 4687.
- Qin, Q.; Wei, F.; Zhang, J.; Li, B. miR-134 suppresses the migration and invasion of non-small cell lung cancer by targeting ITGB1. Oncol. Rep. 2017, 37, 823–830.
- Chen, L.; Qian, H.; Xue, J.; Zhang, J.; Chen, H. MicroRNA-152 regulates insulin secretion and pancreatic β cell proliferation by targeting PI3Kα. Mol. Med. Rep. 2018, 18, 4113–4121.
- Godlewski, J.; Krichevsky, A.M.; Johnson, M.D.; Chiocca, E.A.; Bronisz, A. Belonging to a network—microRNAs, extracellular vesicles, and the glioblastoma microenvironment. Neuro Oncol. 2014, 17, 652–662.
- Saadatpour, L.; Fadaee, E.; Fadaei, S.; Mansour, R.N.; Mohammadi, M.; Mousavi, S.M.; Goodarzi, M.; Verdi, J.; Mirzaei, H. Glioblastoma: Exosome and microRNA as novel diagnosis biomarkers. Cancer Gene Ther. 2016, 23, 415–418.
- Grimson, A.; Srivastava, M.; Fahey, B.; Woodcroft, B.; Chiang, H.R.; King, N.; Degnan, B.; Rokhsar, D.; Bartel, D.P. Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 2008, 455, 1193–1197.
- Ozata, D.M.; Gainetdinov, I.; Zoch, A.; O'Carroll, D.; Zamore, P.D. PIWI-interacting RNAs: Small RNAs with big functions. Nat. Rev. Genet. 2018, 20, 89–108.
- Peng, Q.; Chiu, P.K.-F.; Wong, C.Y.-P.; Cheng, C.K.-L.; Teoh, J.Y.-C.; Ng, C.-F. Identification of piRNA Targets in Urinary Extracellular Vesicles for the Diagnosis of Prostate Cancer. Diagnostics 2021, 11, 1828.
- Gutschner, T.; Diederichs, S. The hallmarks of cancer. RNA Biol. 2012, 9, 703–719.
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407.
- Li, J.; Long, J.; Zhang, Q.; Shen, H.; Guo, A.-Y.; Ma, Z.; Zhang, G. Hypothalamic long noncoding RNA AK044061 is involved in the development of dietary obesity in mice. Int. J. Obes. 2021, 45, 2638–2647.
- Qian, H.; Chen, L.; Huang, J.; Wang, X.; Ma, S.; Cui, F.; Luo, L.; Ling, L.; Luo, K.; Zheng, G. The lncRNA MIR4435-2HG promotes lung cancer progression by activating β-catenin signalling. Klin. Wochenschr. 2018, 96, 753–764.
- Gu, C.; Zou, S.; He, C.; Zhou, J.; Qu, R.; Wang, Q.; Qi, J.; Zhou, M.; Yan, S.; Ye, Z. Long non-coding RNA CCAT1 promotes colorectal cancer cell migration, invasiveness and viability by upregulating VEGF via negative modulation of microRNA-218. Exp. Ther. Med. 2020, 19, 2543–2550.
- Wang, M.; Zhou, L.; Yu, F.; Zhang, Y.; Li, P.; Wang, K. The functional roles of exosomal long non-coding RNAs in cancer. Exp. 2019, 76, 2059–2076.
- Li, X.; Yang, L.; Chen, L.-L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442.
- Yang, X.; Li, S.; Wu, Y.; Ge, F.; Chen, Y.; Xiong, Q. The circular RNA CDR1as regulate cell proliferation via TMED2 and TMED10. BMC Cancer 2020, 20, 1–12.
- Yang, G.; Zhang, Y.; Yang, J. Identification of Potentially Functional CircRNA-miRNA-mRNA Regulatory Network in Gastric Carcinoma using Bioinformatics Analysis. Med. Sci. Monit. 2019, 25, 8777–8796.
- Liu, C.-G.; Li, J.; Xu, Y.; Li, W.; Fang, S.-X.; Zhang, Q.; Xin, H.-W.; Ma, Z. Long non-coding RNAs and circular RNAs in tumor angiogenesis: From mechanisms to clinical significance. Mol. Ther. Oncolytics 2021, 22, 336–354.
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478.
- Cook, K.M.; Figg, W.D. Angiogenesis Inhibitors: Current Strategies and Future Prospects. CA Cancer J. Clin. 2010, 60, 222–243.
- Ma, Z.; Wang, L.Z.; Cheng, J.-T.; Lam, W.S.T.; Ma, X.; Xiang, X.; Wong, A.L.-A.; Goh, B.C.; Gong, Q.; Sethi, G.; et al. Targeting Hypoxia-Inducible Factor-1-Mediated Metastasis for Cancer Therapy. Antioxidants Redox Signal. 2021, 34, 1484–1497.
- Peng, T.; Deng, X.; Tian, F.; Li, Z.; Jiang, P.; Zhao, X.; Chen, G.; Chen, Y.; Zheng, P.; Li, D.; et al. The interaction of LOXL2 with GATA6 induces VEGFA expression and angiogenesis in cholangiocarcinoma. Int. J. Oncol. 2019, 55, 657–670.
- De Bock, K.; Mazzone, M.; Carmeliet, P. Antiangiogenic therapy, hypoxia, and metastasis: Risky liaisons, or not? Nat. Rev. Clin. Oncol. 2011, 8, 393–404.
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370.
- Ma, Z.; Xiang, X.; Li, S.; Xie, P.; Gong, Q.; Goh, B.-C.; Wang, L. Targeting hypoxia-inducible factor-1, for cancer treatment: Recent advances in developing small-molecule inhibitors from natural compounds. Semin. Cancer Biol. 2020, 80, 379–390.
- Jayson, G.C.; Hicklin, D.J.; Ellis, L.M. Antiangiogenic therapy—evolving view based on clinical trial results. Nat. Rev. Clin. Oncol. 2012, 9, 297–303.
- Kim, D.H.; Park, S.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Sung, K.J.; Sung, Y.H.; Choi, C.-M.; Yun, M.; Yi, Y.-S.; et al. Tumor-derived exosomal miR-619-5p promotes tumor angiogenesis and metastasis through the inhibition of RCAN1.4. Cancer Lett. 2020, 475, 2–13.
- Zhang, C.; Luo, Y.; Cao, J.; Wang, X.; Miao, Z.; Shao, G. Exosomal lncRNA FAM225A accelerates esophageal squamous cell carcinoma progression and angiogenesis via sponging miR-206 to upregulate NETO2 and FOXP1 expression. Cancer Med. 2020, 9, 8600–8611.
- Xie, M.; Yu, T.; Jing, X.; Ma, L.; Fan, Y.; Yang, F.; Ma, P.; Jiang, H.; Wu, X.; Shu, Y.; et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol. Cancer 2020, 19, 1–22.
- Deng, T.; Zhang, H.; Yang, H.; Wang, H.; Bai, M.; Sun, W.; Wang, X.; Si, Y.; Ning, T.; Zhang, L.; et al. Exosome miR-155 Derived from Gastric Carcinoma Promotes Angiogenesis by Targeting the c-MYB/VEGF Axis of Endothelial Cells. Mol. Ther. Nucleic Acids 2020, 19, 1449–1459.
- Zhou, Z.; Zhang, H.; Deng, T.; Ning, T.; Liu, R.; Liu, D.; Bai, M.; Ying, G.; Ba, Y. Exosomes Carrying MicroRNA-155 Target Forkhead Box O3 of Endothelial Cells and Promote Angiogenesis in Gastric Cancer. Mol. Ther. Oncolytics 2019, 15, 223–233.
- Yang, H.; Zhang, H.; Ge, S.; Ning, T.; Bai, M.; Li, J.; Li, S.; Sun, W.; Deng, T.; Zhang, L.; et al. Exosome-Derived miR-130a Activates Angiogenesis in Gastric Cancer by Targeting C-MYB in Vascular Endothelial Cells. Mol. Ther. 2018, 26, 2466–2475.
- Bai, M.; Li, J.; Yang, H.; Zhang, H.; Zhou, Z.; Deng, T.; Zhu, K.; Ning, T.; Fan, Q.; Ying, G.; et al. miR-135b Delivered by Gastric Tumor Exosomes Inhibits FOXO1 Expression in Endothelial Cells and Promotes Angiogenesis. Mol. Ther. 2019, 27, 1772–1783.
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757.
- Du, J.; Liang, Y.; Li, J.; Zhao, J.-M.; Lin, X.-Y. Gastric Cancer Cell-Derived Exosomal microRNA-23a Promotes Angiogenesis by Targeting PTEN. Front. Oncol. 2020, 10, 326.
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene 2017, 36, 4929–4942.
- Moh-Moh-Aung, A.; Fujisawa, M.; Ito, S.; Katayama, H.; Ohara, T.; Ota, Y.; Yoshimura, T.; Matsukawa, A. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci. Rep. 2020, 10, 10418.
- Zeng, Z.; Li, Y.; Pan, Y.; Lan, X.; Song, F.; Sun, J.; Zhou, K.; Liu, X.; Ren, X.; Wang, F.; et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat. Commun. 2018, 9, 1–14.
- Hu, H.-Y.; Yu, C.-H.; Zhang, H.-H.; Zhang, S.-Z.; Yu, W.-Y.; Yang, Y.; Chen, Q. Exosomal miR-1229 derived from colorectal cancer cells promotes angiogenesis by targeting HIPK2. Int. J. Biol. Macromol. 2019, 132, 470–477.
- Shang, A.; Wang, X.; Gu, C.; Liu, W.; Sun, J.; Zeng, B.; Chen, C.; Ji, P.; Wu, J.; Quan, W.; et al. Exosomal miR-183-5p promotes angiogenesis in colorectal cancer by regulation of FOXO1. Aging 2020, 12, 8352–8371.
- Lawson, J.; Dickman, C.; Towle, R.; Jabalee, J.; Javer, A.; Garnis, C. Extracellular vesicle secretion of miR-142-3p from lung adenocarcinoma cells induces tumor promoting changes in the stroma through cell-cell communication. Mol. Carcinog. 2018, 58, 376–387.
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.A.; Jian, S.-F.; Lin, Y.-S.; Pan, Y.-C.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung-Cancer-Derived Extracellular Vesicle MicroRNA-103a Increases the Oncogenic Effects of Macrophages by Targeting PTEN. Mol. Ther. 2018, 26, 568–581.
- Pontis, F.; Roz, L.; Mensah, M.; Segale, M.; Moro, M.; Bertolini, G.; Petraroia, I.; Centonze, G.; Ferretti, A.M.; Suatoni, P.; et al. Circulating extracellular vesicles from individuals at high-risk of lung cancer induce pro-tumorigenic conversion of stromal cells through transfer of miR-126 and miR-320. J. Exp. Clin. Cancer Res. 2021, 40, 1–16.
- Masoumi-Dehghi, S.; Babashah, S.; Sadeghizadeh, M. microRNA-141-3p-containing small extracellular vesicles derived from epithelial ovarian cancer cells promote endothelial cell angiogenesis through activating the JAK/STAT3 and NF-κB signaling pathways. J. Cell Commun. Signal. 2020, 14, 233–244.
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220.
- Lu, J.; Liu, Q.-H.; Wang, F.; Tan, J.-J.; Deng, Y.-Q.; Peng, X.-H.; Liu, X.; Zhang, B.; Xu, X.; Li, X.-P. Exosomal miR-9 inhibits angiogenesis by targeting MDK and regulating PDK/AKT pathway in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 2018, 37, 1–12.
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 1–16.
- Bao, L.; You, B.; Shi, S.; Shan, Y.; Zhang, Q.; Yue, H.; Zhang, J.; Zhang, W.; Shi, Y.; Liu, Y.; et al. Metastasis-associated miR-23a from nasopharyngeal carcinoma-derived exosomes mediates angiogenesis by repressing a novel target gene TSGA10. Oncogene 2018, 37, 2873–2889.
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351.
- Lin, X.-J.; Fang, J.-H.; Yang, X.-J.; Zhang, C.; Yuan, Y.; Zheng, L.; Zhuang, S.M. Hepatocellular carcinoma cell-secreted exosomal microRNA-210 promotes angiogenesis in vitro and in vivo. Mol. Ther. Nucleic Acids 2018, 11, 243–252.
- Wang, Z.-F.; Liao, F.; Wu, H.; Dai, J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J. Exp. Clin. Cancer Res. 2019, 38, 1–15.
- Shang, D.; Xie, C.; Hu, J.; Tan, J.; Yuan, Y.; Liu, Z.; Yang, Z. Pancreatic cancer cell–derived exosomal microRNA-27a promotes angiogenesis of human microvascular endothelial cells in pancreatic cancer via BTG2. J. Cell. Mol. Med. 2019, 24, 588–604.
- Yang, Y.; Guo, Z.; Chen, W.; Wang, X.; Cao, M.; Han, X.; Zhang, K.; Teng, B.; Cao, J.; Wu, W.; et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Mol. Ther. 2020, 29, 1226–1238.
- Wu, F.; Li, F.; Lin, X.; Xu, F.; Cui, R.-R.; Zhong, J.-Y.; Zhu, T.; Shan, S.-K.; Liao, X.-B.; Yuan, L.-Q.; et al. Exosomes increased angiogenesis in papillary thyroid cancer microenvironment. Endocr. Relat. Cancer 2019, 26, 525–538.
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.J.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell. Oncol. 2017, 40, 457–470.
- Zhuang, H.; Wang, H.; Yang, H.; Li, H. Exosome-Encapsulated MicroRNA-21 from Esophageal Squamous Cell Carcinoma Cells Enhances Angiogenesis of Human Umbilical Venous Endothelial Cells by Targeting SPRY1. Cancer Manag. Res. 2020, 12, 10651–10667.
- Zhou, Y.; Ren, H.; Dai, B.; Li, J.; Shang, L.; Huang, J.; Shi, X. Hepatocellular carcinoma-derived exosomal miRNA-21 contributes to tumor progression by converting hepatocyte stellate cells to cancer-associated fibroblasts. J. Exp. Clin. Cancer Res. 2018, 37, 324.
- Wang, Y.; Lu, J.; Chen, L.; Bian, H.; Hu, J.; Li, D.; Xia, C.; Xu, H. Tumor-Derived EV-Encapsulated miR-181b-5p Induces Angiogenesis to Foster Tumorigenesis and Metastasis of ESCC. Mol. Ther. Nucleic Acids 2020, 20, 421–437.
- Yao, X.; Xie, L.; Zeng, Y. MiR-9 Promotes Angiogenesis via Targeting on Sphingosine-1- Phosphate Receptor 1. Front. Cell Dev. Biol. 2020, 8, 755.
- Zhang, X.; Wang, Y.; Wang, X.; Zou, B.; Mei, J.; Peng, X.; Wu, Z. Extracellular vesicles-encapsulated microRNA-10a-5p shed from cancer-associated fibroblast facilitates cervical squamous cell carcinoma cell angiogenesis and tumorigenicity via Hedgehog signaling pathway. Cancer Gene Ther. 2020, 28, 529–542.
- Zhang, Y.; Meng, W.; Yue, P.; Li, X. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J. Exp. Clin. Cancer Res. 2020, 39, 1–20.
- Cheng, Y.; Dai, X.; Yang, T.; Zhang, N.; Liu, Z.; Jiang, Y. Low Long Noncoding RNA Growth Arrest-Specific Transcript 5 Expression in the Exosomes of Lung Cancer Cells Promotes Tumor Angiogenesis. J. Oncol. 2019, 2019, 1–13.
- Lang, H.-L.; Hu, G.-W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.-H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017, 38, 785–798.
- Lang, H.-L.; Hu, G.-W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.-M.; Wu, L.; Xu, G.-H. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med Pharmacol. Sci. 2017, 21, 959–972.
- Cheng, C.; Zhang, Z.; Cheng, F.; Shao, Z. Exosomal lncRNA RAMP2-AS1 Derived from Chondrosarcoma Cells Promotes Angiogenesis Through miR-2355-5p/VEGFR2 Axis. Onco. Targets Ther. 2020, 13, 3291–3301.
- Li, Y.; Lin, S.; Xie, X.; Zhu, H.; Fan, T.; Wang, S. Highly enriched exosomal lncRNA OIP5-AS1 regulates osteosarcoma tumor angiogenesis and autophagy through miR-153 and ATG5. Am. J. Transl. Res. 2021, 13, 4211–4223.
- Guo, Z.; Wang, X.; Yang, Y.; Chen, W.; Zhang, K.; Teng, B.; Huang, C.; Zhao, Q.; Qiu, Z. Hypoxic Tumor-Derived Exosomal Long Noncoding RNA UCA1 Promotes Angiogenesis via miR-96-5p/AMOTL2 in Pancreatic Cancer. Mol. Ther. Nucleic Acids 2020, 22, 179–195.
- Fang, X.; Cai, Y.; Xu, Y.; Zhang, H. Exosome-mediated lncRNA SNHG11 regulates angiogenesis in pancreatic carcinoma through miR-324-3p/VEGFA axis. Cell Biol. Int. 2022, 46, 106–117.
- Dai, G.; Yang, Y.; Liu, S.; Liu, H. Hypoxic Breast Cancer Cell-Derived Exosomal SNHG1 Promotes Breast Cancer Growth and Angiogenesis via Regulating miR-216b-5p/JAK2 Axis. Cancer Manag. Res. 2022, 14, 123–133.
- Kong, X.; Li, J.; Li, Y.; Duan, W.; Qi, Q.; Wang, T.; Yang, Q.; Du, L.; Mao, H.; Wang, C. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021, 12, 1–13.
- Qiu, J.-J.; Lin, X.-J.; Tang, X.-Y.; Zheng, T.-T.; Lin, Y.-Y.; Hua, K.-Q. Exosomal Metastasis-Associated Lung Adenocarcinoma Transcript 1 Promotes Angiogenesis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973.
- Lei, L.; Mou, Q. Exosomal taurine up-regulated 1 promotes angiogenesis and endothelial cell proliferation in cervical cancer. Cancer Biol. Ther. 2020, 21, 717–725.
- You, L.-N.; Tai, Q.-W.; Xu, L.; Hao, Y.; Guo, W.-J.; Zhang, Q.; Tong, Q.; Zhang, H.; Huang, W.-K. Exosomal LINC00161 promotes angiogenesis and metastasis via regulating miR-590-3p/ROCK axis in hepatocellular carcinoma. Cancer Gene Ther. 2021, 28, 719–736.
- Conigliaro, A.; Costa, V.; Dico, A.L.; Saieva, L.; Buccheri, S.; Dieli, F.; Manno, M.; Raccosta, S.; Mancone, C.; Tripodi, M.; et al. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol. Cancer 2015, 14, 155.
- Huang, X.-Y.; Huang, Z.-L.; Huang, J.; Xu, B.; Huang, X.-Y.; Xu, Y.-H.; Zhou, J.; Tang, Z.-Y. Exosomal circRNA-100338 promotes hepatocellular carcinoma metastasis via enhancing invasiveness and angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 20.
- Hu, K.; Li, N.; Li, J.; Chen, Z.; Wang, J.; Sheng, L. Exosome circCMTM3 promotes angiogenesis and tumorigenesis of hepatocellular carcinoma through miR-3619-5p/SOX9. Hepatol. Res. 2021, 51, 1139–1152.
- Bai, L.; Gao, Z.; Jiang, A.; Ren, S.; Wang, B. Circular noncoding RNA circ_0007334 sequestrates miR-577 to derepress KLF12 and accelerate colorectal cancer progression. AntiCancer Drugs 2022, 33, e409–e422.
- Zeng, W.; Liu, Y.; Li, W.; Zhu, J. CircFNDC3B sequestrates miR-937-5p to derepress TIMP3 and inhibit colorectal cancer progression. Mol. Oncol. 2020, 14, 2960–2984.
- Li, Y.; Chen, J.; Chen, Z.; Xu, X.; Weng, J.; Zhang, Y.; Mo, Y.; Liu, Y.; Wang, J.; Ke, Y. CircGLIS3 Promotes High-Grade Glioma Invasion via Modulating Ezrin Phosphorylation. Front. Cell Dev. Biol. 2021, 9.
- Li, B.; Hong, J.; Hong, M.; Wang, Y.; Yu, T.; Zang, S.; Wu, Q. piRNA-823 delivered by multiple myeloma-derived extracellular vesicles promoted tumorigenesis through re-educating endothelial cells in the tumor environment. Oncogene 2019, 38, 5227–5238.
- Jiang, L.; Gu, Y.; Du, Y.; Liu, J. Exosomes: Diagnostic Biomarkers and Therapeutic Delivery Vehicles for Cancer. Mol. Pharm. 2019, 16, 3333–3349.
- Zhang, W.; Su, X.; Li, S.; Liu, Z.; Wang, Q.; Zeng, H. Low serum exosomal miR-484 expression predicts unfavorable prognosis in ovarian cancer. Cancer Biomark. 2020, 27, 485–491.
- Liu, C.-J.; Xie, G.-Y.; Miao, Y.-R.; Xia, M.; Wang, Y.; Lei, Q.; Zhang, Q.; Guo, A.-Y. EVAtlas: A comprehensive database for ncRNA expression in human extracellular vesicles. Nucleic Acids Res. 2021, 50, D111–D117.
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741.
- Li, J.; Zhang, G.; Liu, C.-G.; Xiang, X.; Le, M.T.; Sethi, G.; Wang, L.; Goh, B.-C.; Ma, Z. The potential role of exosomal circRNAs in the tumor microenvironment: Insights into cancer diagnosis and therapy. Theranostics 2022, 12, 87–104.
- Maishi, N.; Hida, K. Tumor endothelial cells accelerate tumor metastasis. Cancer Sci. 2017, 108, 1921–1926.
- Ogata-Kawata, H.; Izumiya, M.; Kurioka, D.; Honma, Y.; Yamada, Y.; Furuta, K.; Gunji, T.; Ohta, H.; Okamoto, H.; Sonoda, H.; et al. Circulating Exosomal microRNAs as Biomarkers of Colon Cancer. PLoS ONE 2014, 9, e92921.
- Pan, L.; Liang, W.; Fu, M.; Huang, Z.-H.; Li, X.; Zhang, W.; Zhang, P.; Qian, H.; Jiang, P.-C.; Xu, W.-R.; et al. Exosomes-mediated transfer of long noncoding RNA ZFAS1 promotes gastric cancer progression. J. Cancer Res. Clin. Oncol. 2017, 143, 991–1004.
- Xu, H.; Chen, Y.; Dong, X.; Wang, X. Serum Exosomal Long Noncoding RNAs ENSG00000258332.1 and LINC00635 for the Diagnosis and Prognosis of Hepatocellular Carcinoma. Cancer Epidemiol. Biomark. Prev. 2018, 27, 710–716.
- Xian, J.; Su, W.; Liu, L.; Rao, B.; Lin, M.; Feng, Y.; Qiu, F.; Chen, J.; Zhou, Q.; Zhao, Z.; et al. Identification of Three Circular RNA Cargoes in Serum Exosomes as Diagnostic Biomarkers of Non–Small-Cell Lung Cancer in the Chinese Population. J. Mol. Diagn. 2020, 22, 1096–1108.
- Chen, X.; Chen, R.-X.; Wei, W.-S.; Li, Y.-H.; Feng, Z.-H.; Tan, L.; Chen, J.-W.; Yuan, G.-J.; Chen, S.-L.; Guo, S.-J.; et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial–Mesenchymal Transition. Clin. Cancer Res. 2018, 24, 6319–6330.
- Zhou, Y.; Zhou, G.; Tian, C.; Jiang, W.; Jin, L.; Zhang, C.; Chen, X. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscip. Rev. RNA 2016, 7, 758–771.
- Teo, A.; Xiang, X.; Le, M.; Wong, A.; Zeng, Q.; Wang, L.; Goh, B.-C. Tiny miRNAs Play a Big Role in the Treatment of Breast Cancer Metastasis. Cancers 2021, 13, 337.
- Zhang, H.; Bai, M.; Deng, T.; Liu, R.; Wang, X.; Qu, Y.; Duan, J.; Zhang, L.; Ning, T.; Ge, S.; et al. Cell-derived microvesicles mediate the delivery of miR-29a/c to suppress angiogenesis in gastric carcinoma. Cancer Lett. 2016, 375, 331–339.
- Wang, J.; Jiang, Q.; Faleti, O.D.; Tsang, C.-M.; Zhao, M.; Wu, G.; Tsao, S.-W.; Fu, M.; Chen, Y.; Ding, T.; et al. Exosomal Delivery of AntagomiRs Targeting Viral and Cellular MicroRNAs Synergistically Inhibits Cancer Angiogenesis. Mol. Ther. Nucleic Acids 2020, 22, 153–165.
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 1–14.
- Chen, S.; Chen, X.; Luo, Q.; Liu, X.; Wang, X.; Cui, Z.; He, A.; He, S.; Jiang, Z.; Wu, N.; et al. Retinoblastoma cell-derived exosomes promote angiogenesis of human vesicle endothelial cells through microRNA-92a-3p. Cell Death Dis. 2021, 12, 1–11.




