Most C4 plants that naturally occur in tropical or subtropical climates, in high light environments, had to evolve a series of adaptations of photosynthesis that allowed them to grow under these conditions. Some mechanisms that function under changing light conditions, particularly in high light intensity, are universal and are also found in C3 plants. However, some are modified in C4 plants to provide more efficient CO2 assimilation. The close relationship between the light phase of photosynthesis and the enzymatic reactions in chloroplasts, and the associated demand for ATP and NADPH, results that in C4 plants the linear and cyclic electron transport operate in a different ratio in the chloroplasts of mesophyll (M) and bundle sheath (BS) cells. In addition, differences in the intensity of light reaching M and BS chloroplasts and in the thylakoid structure (granal and agranal) will affect the processes of the redistribution of excitation energy between photosystems and the dissipation of its excess. Therefore, it can be assumed that, in the M chloroplasts, because of increased incoming light energy, the mechanisms related to the dissipation of excess energy must function better than in BS chloroplasts to prevent photosystems from photoinhibition and, in consequence, from a decrease in ATP and NADPH. On the other hand, BS chloroplasts, which receive less light energy, must have better functioning mechanisms that allow for its efficient use.
1. Diversity of C4 Photosynthesis
Photosynthesis is a complex metabolic process in which solar energy is utilized to convert atmospheric carbon dioxide (CO
2) into organic compounds. Traditionally, it is divided into two phases: (1) the light energy captured by the pigment–protein complexes is exchanged into energy-rich bonds of molecules such as ATP and NADPH, and (2) when ATP and NADPH drive the fixation of CO
2 in carbohydrates. Approximately 85% of all higher plants use the C3 photosynthetic pathway
[1]. In this process, the first step of CO
2 fixation is catalyzed by ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) and two three-carbon molecules (3-phosphoglyceric acid) are produced by carboxylation of ribulose-1,5-bisphosphate. However, RuBisCO also shows oxygenase activity, which leads to carbon loss by photorespiration. Plants have evolved mechanisms that allow them to concentrate CO
2 at the RuBisCO site and thus eliminate or reduce the oxygenase activity of RuBisCO. One of them is C4 photosynthesis, a pathway described in the 1960s
[2][3][4]. In this type of photosynthesis (
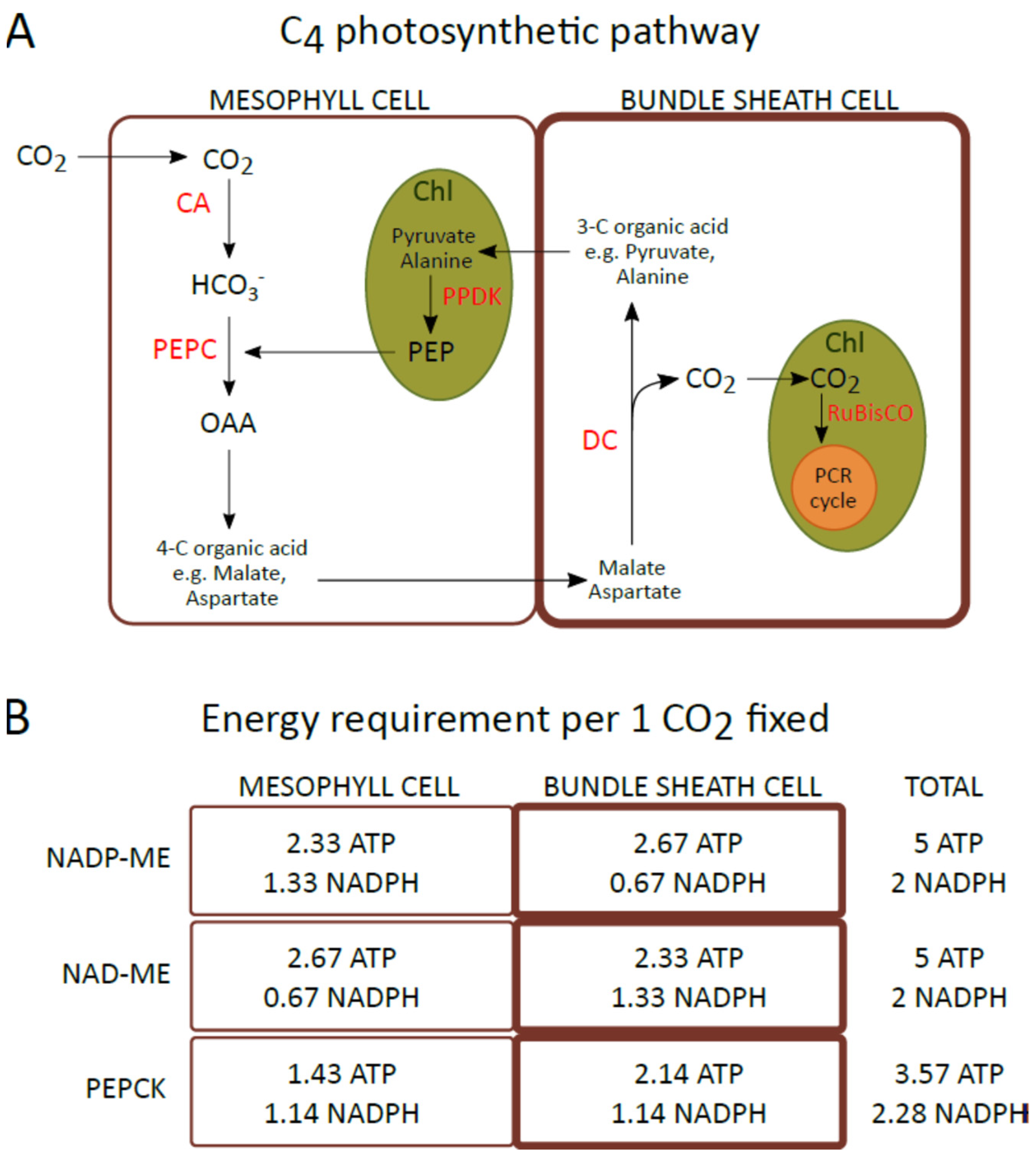
Figure 1A), the assimilation and reduction of CO
2 are spatially separated and catalyzed by two different enzymes. Carbon dioxide assimilation occurs in the cytoplasm of mesophyll (M) cells, where CO
2 is initially converted to HCO
3−, which is then incorporated into phosphoenolpyruvate (PEP) to form oxaloacetate (OAA) by phosphoenolpyruvate carboxylase (PEPC). Then, OAA is converted to malate or aspartate, and these carboxylic acids are transported to bundle sheath (BS) cells, where they are decarboxylated. The CO
2 released is then assimilated by RuBisCO (present only in the chloroplasts of BS cells) through the Calvin–Benson cycle
[5]. Due to differences in a type of carboxylic acid transferred to BS cells and the main enzyme responsible for the decarboxylation in these cells, and regeneration of the CO
2 acceptor in M cells, three biochemical subtypes of C4 photosynthesis are recognized: NADP-ME, NAD-ME, and PEPCK or PCK
[5][6][7]. In the most common NADP-ME subtype, malate is transferred from the mesophyll to bundle sheath cells and CO
2 is released into the chloroplasts of BS by the NADP-dependent malic enzyme (NADP-ME). However, in the NAD-ME subtype, aspartate is the main carboxylic acid that is transported from M to BS cells, and decarboxylation is catalyzed by mitochondrial NAD-dependent malic enzyme (NAD-ME). In the third PEPCK subtype, both aspartate and malate are transferred to BS cells, where aspartate is converted to OAA in the cytosol. Then, it is decarboxylated by PEP carboxykinase (PEPCK or PCK). Moreover, malate synthesized in the mesophyll chloroplast is transported into BS mitochondria, where decarboxylation catalyzed by NAD-ME occurs. Furthermore, although C4 photosynthesis has been classified into three subtypes, accumulating evidence indicates that many C4 plants use a combination of organic acids and decarboxylases during CO
2 fixation, and the C4-type categories are not rigid
[8]. The C4 metabolic cycle consumes more energy than C3 photosynthesis. Fixation of 1 CO
2 requires 5 ATP and 2 NADPH for plants driving the NADP-ME and NAD-ME photosynthesis subtypes and 3.6 ATP and 2.3 NADPH for the PEPCK subtype (
Figure 1B), whereas for the C3 cycle this energy demand is 3 ATP and 2 NADPH
[9][10].
Figure 1. (
A) Schematic describing the general metabolic pathway of C4 photosynthesis. (
B) The requirement for ATP and NADPH per fixed CO
2 in mesophyll and bundle sheath cells of plants representing the NADP-ME, NAD-ME, and PEPCK subtypes of C4 photosynthesis. ATP and NADPH requirements estimated by Edwards and Voznesenskaya
[9]. CA: carbonic anhydrase; Chl: chloroplast; DC: decarboxsylase; OAA: oxaloacetate; PCR cycle: photosynthetic carbon reduction cycle; PEP: phosphoenolpyruvate; PEPC: phosphoenolpyruvate carboxylase; PPDK: pyruvate, phosphate dikinase; RuBisCO: ribulose-1,5-bisphosphate carboxylase/oxygenase.
Separation of assimilation and CO
2 reduction into two cell types involves several adaptations in the anatomy and ultrastructure of the leaf
[5]. This special leaf anatomy in the C4 plant is called Kranz and characterizes thick-walled (containing suberin) large parenchyma cells (BS cells) that tightly surround vascular bundles, while mesophyll cells, with much thinner walls, are located between the leaf epidermis and BS cells
[11]. In addition, mesophyll and BS cells are connected by a dense network of plasmodesmata through which an intensive transport of metabolites takes place
[5]. Furthermore, the M and BS chloroplasts in species belonging to different subtypes of C4 photosynthesis exhibit structural dimorphism. In the NADP-ME subtype, chloroplasts localized in M cells have grana, whereas BS chloroplasts are deficient in these structures or grana stacks are rare
[12][13]. In contrast, in the NAD-ME type, mesophyll chloroplasts are more deficient in grana than the BS chloroplast
[14][15]. In the PEPCK subtype, M and BS chloroplasts demonstrate a similar pattern of granal development
[13][16]. This dimorphism is related to differences in the need for NADPH and ATP between the chloroplasts of the mesophyll and bundle sheath cells to support C4 photosynthesis
[9].
This spatial separation of CO
2 assimilation and reduction allows for up to a tenfold increase in the CO
2 concentration in BS cells compared to the natural concentration of this gas in the air
[17]. The maintenance of the high concentration of CO
2 at the RuBisCO site is also related in part to the lack or low levels of carbonic anhydrase. This prevents a rapid conversion of carbon dioxide into a carbonate anion, which is not the substrate for RuBisCO
[18]. The mechanism of CO
2 concentration, the higher CO
2 assimilation capacity, and the reduction of stomatal conductance help C4 plants maintain higher rates of carbon gain compared to C3 plants
[19][20], 50–300% higher water use efficiency
[20], and higher nitrogen use efficiency
[21][22]. These advantages allow C4 plants to grow at high light intensities, high temperatures, and under arid conditions, where the photorespiration process can reduce C3 photosynthesis by up to 30%
[23].
Photosynthesis of C4 is a relatively recent innovation that has evolved independently more than sixty times during the last 30 million years of land plant evolution
[24]. The significant differences in anatomy, biochemistry, and physiology between different C4 species are based on various genetic changes that include simple modifications of the molecular sequence of genes involved in photosynthesis, alterations of regulatory elements, gene duplications, or subcellular retargeting of proteins, and lateral transfer of genetic material. A good example of simple molecular sequence modification is PEP carboxylase, in which 21 different amino acid positions have been elucidated in grasses
[25]. Larger changes in DNA sequences are also frequent and include duplication of entire genomes or genes involved in the C4 cycle
[25][26][27]. Interestingly, it is currently thought that the duplication of genes encoding proteins of the core C4 cycle generally occurred before the origin of C4 photosynthesis and this may have been a contributing factor that allowed the evolution of C4 plants through the acquisition of new functions by duplicated genes. However, genetic modifications related to the evolution of C4 photosynthesis are not fully understood and are still intensively studied.
2. Ways of Light Energy Utilization: Balanced Distribution between Photosystems and Emission of Excess Energy as a Heat
Environmental factors, especially light intensity and quality during growth, can cause changes in pigment–protein complexes involved in the light reactions of photosynthesis. In some cases, light can cause photoinhibition, which is associated with the overproduction of reactive oxygen species (ROS) and damage of thylakoid membrane components
[28]. The plant response may be more complex when there are several environmental factors, which in the case of C4 plants is quite natural due to their ability to grow in hot, and dry conditions with high light intensity
[29][30].
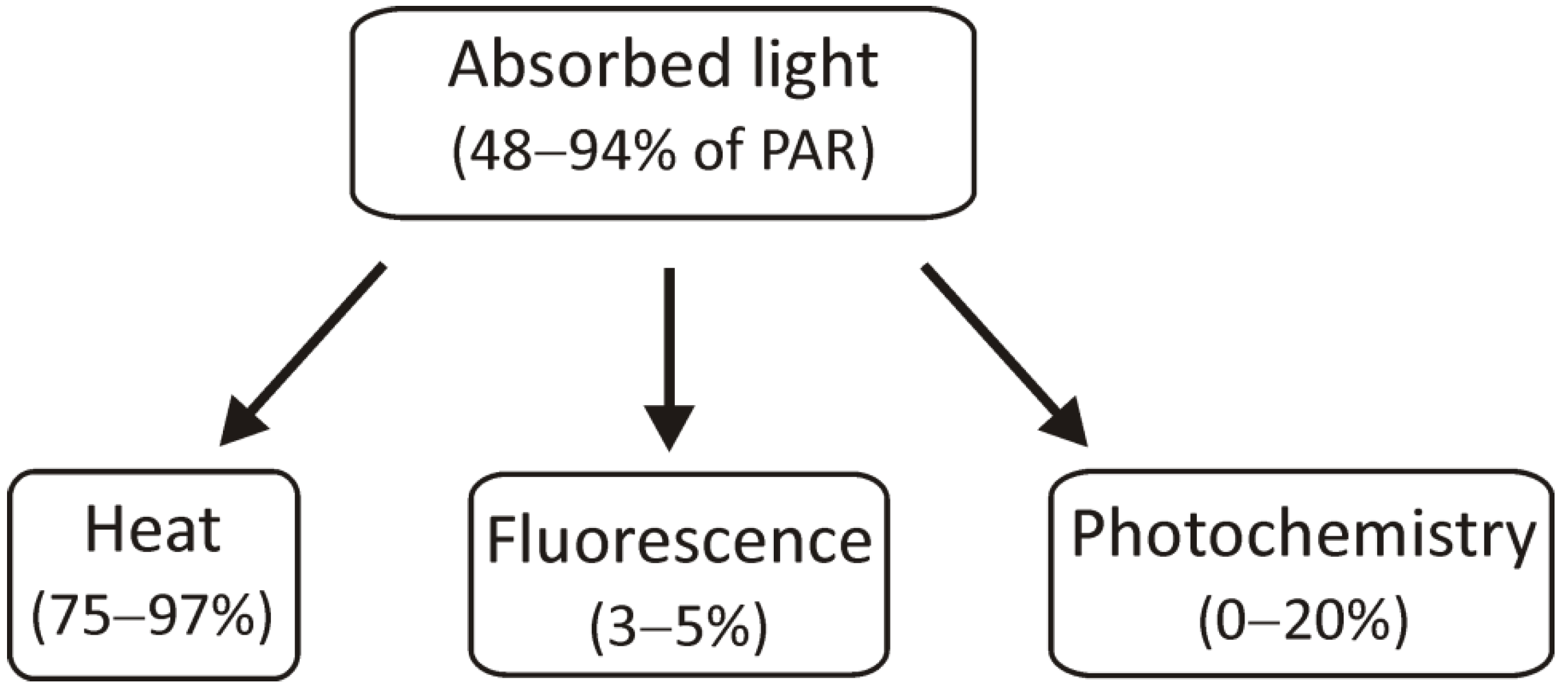
The light energy that has been absorbed by chlorophyll molecules can be used in three ways: it can be used for photochemical reactions, its excess can be dissipated as heat, or it can be transferred as light, i.e., fluorescence (Figure 2).
Figure 2. The scheme presents the use of absorbed light energy for photochemical reactions, heat dissipation, and radiation by fluorescence. PAR: photosynthetically active radiation.
Changes in light intensity under natural conditions occur rapidly, and the amount of light absorbed often exceeds the efficiency of photosynthetic reactions. It should be noted that photosynthetic light reactions play a crucial role due to the products of linear (LET) and cyclic electron transport (CET), that is, the supply of ATP and NADPH, necessary for the assimilation of CO
2 in the Calvin cycle
[31]. Mechanisms that function as safety valves in chloroplasts can be divided into those universal and concern not only the protection of C4 plants, but also C3, such as the xanthophyll cycle, heat dissipation, or state transitions, and those more characteristic for C4, such as promoting cyclic electron transport and alternative CET pathways, routes to increase ATP production
[31]. It should be noted that in C4 plants the response to environmental factors can be more complicated due to the existence of different metabolic subtypes (NADP-ME, NAD-ME, PEPCK), different structures of M and BS chloroplasts within these subtypes, and different requirements for ATP and NADPH.
2.1. Elevation of Cyclic Electron Transport Components
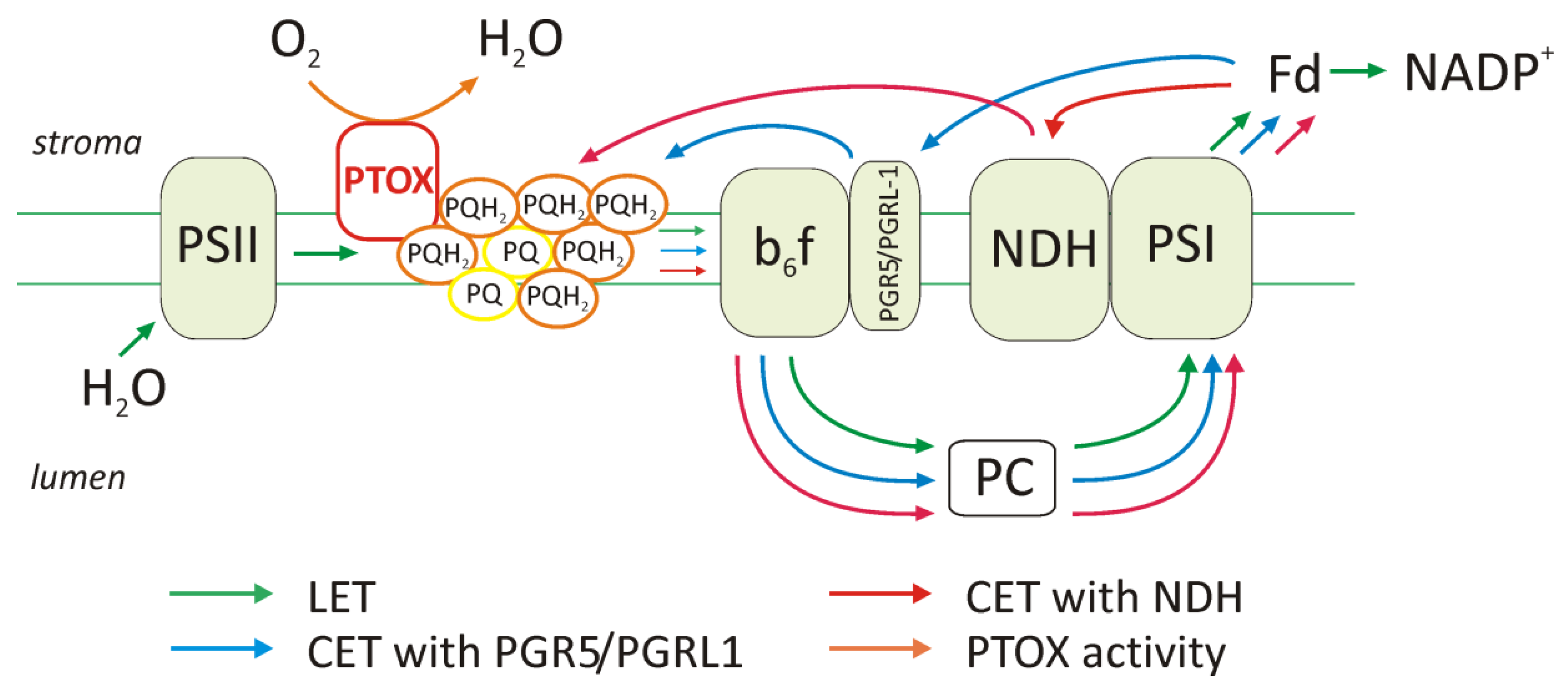
C4 plants have higher cyclic electron transport activity compared to C3, and this increase may have been most important for the production of the additional ATP pool required for C4 metabolism. Studies on the genus Flaveria showed an increase in the content of cyclic electron transport components, such as PGR5 (proton gradient regulation 5), PGRL1 (proton gradient regulation like 1) proteins, and the NDH (NADH dehydrogenase-like) complex in the chloroplast of BS cells during the evolution of C4 photosynthesis (Figure 3). Furthermore, the reduction in the amount of grana or the presence of agranal thylakoid organization, as in the maize BS chloroplasts, was associated with a higher content of components such as PGR5, PGRL1, and NDH. In C4 plants, two extra ATP molecules are required to assimilate each CO2 molecule in the Calvin cycle. As mentioned above (Figure 1), in the NADP-ME subtype, the demand for ATP is higher in chloroplasts of BS than in mesophyll chloroplasts. It is indicated that additional ATP molecules are produced by the cyclic electron transport, which dominates in this type of chloroplast and ensures the generation of a pH gradient across the membrane thylakoid without NADPH production.
Figure 3. The ways of electron transport in the thylakoid membrane under high light intensities and over-reduction of the plastoquinone pool (PQ). The scheme shows linear electron transport (LET), cyclic electron transport (CET), which takes place with the participation of the proteins PGR5 and PGRL1 or/and the NDH complex, and the activity of PTOX in the chlororespiration process. The content of complexes and the activity of the electron transport pathway will differ in the M and BS chloroplasts in particular metabolic subtypes and depending on environmental conditions.
2.2. Function of PTOX Protein and Chlororespiration
In addition to transmembrane complexes, such as PSI, cytochrome
b6f, or PSII, there are also additional components that are important in electron transport. These include the NADH chloroplast dehydrogenase complex (described above) and the plastid terminal oxidase (PTOX)
[32] (
Figure 3). The approximately 40 kDa PTOX protein is similar to mitochondrial alternative oxidase (AOX) and is a plastoquinone:O
2 oxidoreductase, commonly found in plants. It is located on the stromal side of the thylakoid membrane between PSII and the cytochrome
b6f complex
[32]. It oxidizes the plastoquinone pool and, by donating electrons to oxygen, leads to the formation of a water molecule. This protects the plastoquinone against excessive reduction
[33]. PTOX accounts for 1% of the PSII content in
Arabidopsis thaliana, and it is located in the non-appressed regions of thylakoids where PSI is dominant
[34].
There is little data on PTOX activity in individual C4 subtypes in response to high light intensities. This protein has been shown to be involved in tolerance to salt stress in a representative of PEPCK
Spartina alterniflora, which is a halophyte. PTOX may provide an alternative pathway for the protection of this species against over-reduction and minimize or avoid damage to both photosystems (PSI and PSII)
[35]. The
scholars suggest that
Spartina alterniflora gained increased salt tolerance because of increased electron flow through PTOX, which can be a major sink of electrons in salt stress and functions as a safety valve to protect photosystems from over-reduction, in contrast to the response observed in the glycophyte
Setaria viridis (NADP-ME). These experiments were carried out on whole leaves, so they do not provide information on the content and activity of PTOX individually in the M and BS chloroplasts.
2.3. Changes in the Amount of Thylakoid Complexes
The LHCII antenna system is one of the complexes in thylakoid membranes whose content changes under conditions of varying light intensities
[36]. Adaptation to high light intensities in maize is a tightly coordinated regulation of the components/activity of the light reaction in both mesophyll and bundle sheath chloroplasts
[37]. Under different light intensities, both the content of individual proteins and the arrangement of the complexes can change. Low light intensity promotes the development of antenna systems to capture as much light energy as possible, in contrast to high light when LHC systems decrease. When the intensity of light increases during growth, the levels of the PSII and PSI reaction centers, as well as the cytochrome
b6f complex, increase
[37]. It has also been shown that the content of LHCII antenna systems increases under conditions of low light, even in maize BS chloroplasts where the PSII content is reduced.
Light, both its quantity and quality, as well as other environmental factors (e.g., temperature, CO
2 and O
2 concentration, drought, and also phosphate availability) affect the expression of chloroplast genes, which is dependent on the redox state of the chloroplasts. The PQH
2/PQ ratio influences the transcription genes that encode the proteins of the PSI and PSII reaction centers
[38], allowing the photosynthetic apparatus to be adjusted to the actual conditions. Maintaining the oxidized pool of plastoquinone (PQ) by exposure to PSI excitation light or by inhibiting electron transport in PSII was found to activate transcription of the
psbA gene encoding D1—the core protein of PSII. In contrast, when the pool of plastoquinone is in a reduced state (PQH
2), during exposure to PSII excitation light, the transcription of the
psaA and
psaB genes encoding the PSI reaction center proteins is activated. Variable light conditions also influence the rate of the Calvin cycle. Therefore, if the demand for ATP and NADPH and their production in light reactions of photosynthesis changes, the degree of reduction of the plastoquinone pool will also change. This has an impact on the expression of genes encoding core proteins of the photosystems, and this, in turn, may change the proportion of cyclic or linear electron transport under given conditions.
2.4. Photoinhibition and Role of D1 Protein Phosphorylation
Photoinhibition is a phenomenon that leads to a decrease in photosynthetic activity and a reduction in CO
2 assimilation. It is defined as the light-induced inhibition of photosystem II activity
[39] when photosystem II degradation dominates over its repair
[40]. The classic model of photoinhibition assumed the generation of reactive oxygen species by excessive reduction of the plastoquinone pool. The formed reactive oxygen species are responsible for the damage to the PSII reaction center. Currently, many
scholars indicate that PSII repair processes are more sensitive to environmental stresses
[28][41]. Photosystem II is considered to be the most damage-sensitive complex of the thylakoid membrane, which does not mean that PSI is not affected by photoinhibition as well. PSI photoinhibition occurs when the supply of electrons from PSII exceeds the acceptor capacity of PSI
[42], but PSI is effectively protected against damage, for example, by photoinhibition of a certain pool of PSII.
Repair of damaged PSII reaction centers requires the degradation of the D1 protein destroyed during photoinhibition, its de novo synthesis, and reconstruction of the PSII complex. D1 degradation is a multistage process regulated by protein phosphorylation and dephosphorylation, and also by the level of ATP in chloroplasts. The D1 protein, one of the most easily degraded, is phosphorylated under the influence of medium and high intensity of light in the granum of thylakoids by membrane-bound serine threonine kinase. This modification affects both intact and damaged reaction centers, protects against proteolytic degradation, and has no effect on the electron transport rate in PSII
[43]. When irreversible damage of D1 is caused by photoinhibition, this D1 is directed to the thylakoid stroma, where it is dephosphorylated and then degraded
[44].
2.5. State Transitions and Phosphorylation of LHCII
At different light intensities, the migration of LHCII between photosystems is observed in the process called state transitions. The LHCII antenna, and especially the Lhcb2 protein, undergoes reversible phosphorylation, which is crucial for the switching of LHCII between photosystems. The levels of LHCII phosphorylation are lower at high light compared with those under low light conditions. State 1 is traditionally defined as the condition when PSI is preferentially excited and all LHCIIs become associated with PSII. Illumination conditions, which lead to an excess excitation of photosystem II (PSII), compared to photosystem I (PSI), induce a transition to state 2, in which the more absorbed excitation energy is diverted to PSI because the phosphorylated LHCII antennas are associated with PSI
[45]. State transitions act as a mechanism to balance the excitation of the two photosystems under changing light regimes
[46].
In C4 plants where there are differences in the organization of thylakoid membranes in the M and BS chloroplasts, the process may be quite different. Thylakoid membranes are heterogeneous, and while PSII with the LHCII antenna is located in the stacked regions of the grana, the PSI occurs in the stroma lamellae and marginal grana regions. Thus, the number of grana in a given chloroplast type in each metabolic subtype will determine the LHCII content and the amount of PSI to which these antennas can potentially be attached.
2.6. Xanthophyll Cycle and Heat Dissipation
Among several mechanisms in chloroplasts that allow them to function under stress conditions, preventing the generation of ROS is extremely important. One of the protection mechanisms is the quenching of excess energy as a thermal dissipation. This process involves the xanthophyll cycle, related to the conversion of violaxanthin to zeaxanthin
[47] and the protonation of the PsbS protein. Generally, when plants are exposed to high light intensities, violaxanthin is oxidized by violaxanthin de-epoxidase (VDE). This leads to the formation of antheraxanthin, followed by zeaxanthin. Zeaxanthin creates a barrier that prevents overexcitation of the PSII reaction center. The energy from LHCII is dissipated and is not directed to the reaction center. At low light intensities, zeaxanthin epoxidation catalyzed by zeaxanthin epoxidase (ZE) occurs. The content of carotenoids, including xanthophylls participating in the xanthophyll cycle, in M and BS chloroplasts was investigated by the group of Romanowska et al.
[48]. An increase in zeaxanthin content was also observed in
Sorghum bicolor (NADP-ME plant), after exposure to the same light intensity as described above. The amount of zeaxanthin, antheraxanthin, and violaxanthin participating in the xanthophyll cycle was twice as high compared to the control
[49], which may indicate that regardless of the species, the functioning of the xanthophyll cycle is an important element of protection of the photosynthetic apparatus and dissipation of excess energy.
The participation of the LHCII antenna in energy quenching was confirmed in the mesophyll chloroplasts of maize (NADP-ME), where after the high intensity of far-red light, the LHCII were dephosphorylated, detached from the PSI in the stroma lamellae, moved to the grana, and either bound to PSII or formed aggregates which in consequence, lead to induction of the qE parameter
[46]. In M chloroplasts, light is not a factor that limits the production of ATP and NADPH, so the excess light energy is dissipated as heat.