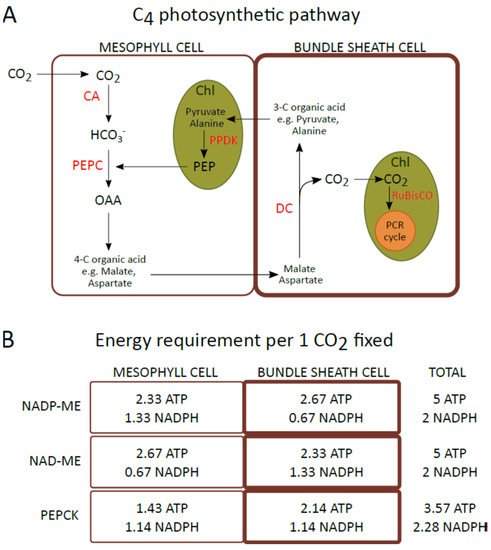
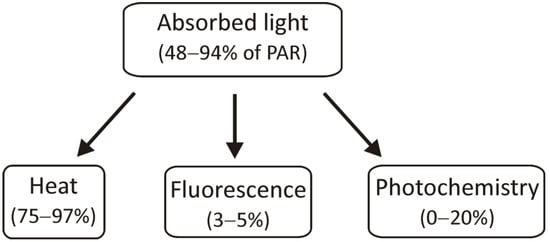
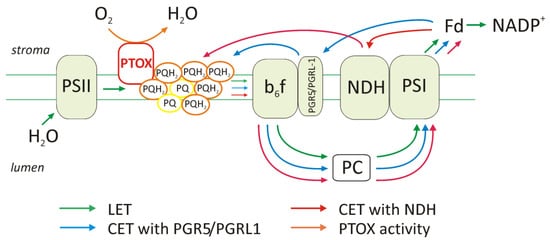
2.3. Changes in the Amount of Thylakoid Complexes
The LHCII antenna system is one of the complexes in thylakoid membranes whose content changes under conditions of varying light intensities
[36]. Adaptation to high light intensities in maize is a tightly coordinated regulation of the components/activity of the light reaction in both mesophyll and bundle sheath chloroplasts
[37]. Under different light intensities, both the content of individual proteins and the arrangement of the complexes can change. Low light intensity promotes the development of antenna systems to capture as much light energy as possible, in contrast to high light when LHC systems decrease. When the intensity of light increases during growth, the levels of the PSII and PSI reaction centers, as well as the cytochrome
b6f complex, increase
[37]. It has also been shown that the content of LHCII antenna systems increases under conditions of low light, even in maize BS chloroplasts where the PSII content is reduced.
Light, both its quantity and quality, as well as other environmental factors (e.g., temperature, CO
2 and O
2 concentration, drought, and also phosphate availability) affect the expression of chloroplast genes, which is dependent on the redox state of the chloroplasts. The PQH
2/PQ ratio influences the transcription genes that encode the proteins of the PSI and PSII reaction centers
[38], allowing the photosynthetic apparatus to be adjusted to the actual conditions. Maintaining the oxidized pool of plastoquinone (PQ) by exposure to PSI excitation light or by inhibiting electron transport in PSII was found to activate transcription of the
psbA gene encoding D1—the core protein of PSII. In contrast, when the pool of plastoquinone is in a reduced state (PQH
2), during exposure to PSII excitation light, the transcription of the
psaA and
psaB genes encoding the PSI reaction center proteins is activated. Variable light conditions also influence the rate of the Calvin cycle. Therefore, if the demand for ATP and NADPH and their production in light reactions of photosynthesis changes, the degree of reduction of the plastoquinone pool will also change. This has an impact on the expression of genes encoding core proteins of the photosystems, and this, in turn, may change the proportion of cyclic or linear electron transport under given conditions.
2.4. Photoinhibition and Role of D1 Protein Phosphorylation
Photoinhibition is a phenomenon that leads to a decrease in photosynthetic activity and a reduction in CO
2 assimilation. It is defined as the light-induced inhibition of photosystem II activity
[39] when photosystem II degradation dominates over its repair
[40]. The classic model of photoinhibition assumed the generation of reactive oxygen species by excessive reduction of the plastoquinone pool. The formed reactive oxygen species are responsible for the damage to the PSII reaction center. Currently, many
scholarauthors indicate that PSII repair processes are more sensitive to environmental stresses
[28][41]. Photosystem II is considered to be the most damage-sensitive complex of the thylakoid membrane, which does not mean that PSI is not affected by photoinhibition as well. PSI photoinhibition occurs when the supply of electrons from PSII exceeds the acceptor capacity of PSI
[42], but PSI is effectively protected against damage, for example, by photoinhibition of a certain pool of PSII.
Repair of damaged PSII reaction centers requires the degradation of the D1 protein destroyed during photoinhibition, its de novo synthesis, and reconstruction of the PSII complex. D1 degradation is a multistage process regulated by protein phosphorylation and dephosphorylation, and also by the level of ATP in chloroplasts. The D1 protein, one of the most easily degraded, is phosphorylated under the influence of medium and high intensity of light in the granum of thylakoids by membrane-bound serine threonine kinase. This modification affects both intact and damaged reaction centers, protects against proteolytic degradation, and has no effect on the electron transport rate in PSII
[43]. When irreversible damage of D1 is caused by photoinhibition, this D1 is directed to the thylakoid stroma, where it is dephosphorylated and then degraded
[44].
2.5. State Transitions and Phosphorylation of LHCII
At different light intensities, the migration of LHCII between photosystems is observed in the process called state transitions. The LHCII antenna, and especially the Lhcb2 protein, undergoes reversible phosphorylation, which is crucial for the switching of LHCII between photosystems. The levels of LHCII phosphorylation are lower at high light compared with those under low light conditions. State 1 is traditionally defined as the condition when PSI is preferentially excited and all LHCIIs become associated with PSII. Illumination conditions, which lead to an excess excitation of photosystem II (PSII), compared to photosystem I (PSI), induce a transition to state 2, in which the more absorbed excitation energy is diverted to PSI because the phosphorylated LHCII antennas are associated with PSI
[45]. State transitions act as a mechanism to balance the excitation of the two photosystems under changing light regimes
[46].
In C4 plants where there are differences in the organization of thylakoid membranes in the M and BS chloroplasts, the process may be quite different. Thylakoid membranes are heterogeneous, and while PSII with the LHCII antenna is located in the stacked regions of the grana, the PSI occurs in the stroma lamellae and marginal grana regions. Thus, the number of grana in a given chloroplast type in each metabolic subtype will determine the LHCII content and the amount of PSI to which these antennas can potentially be attached.
2.6. Xanthophyll Cycle and Heat Dissipation
Among several mechanisms in chloroplasts that allow them to function under stress conditions, preventing the generation of ROS is extremely important. One of the protection mechanisms is the quenching of excess energy as a thermal dissipation. This process involves the xanthophyll cycle, related to the conversion of violaxanthin to zeaxanthin
[47] and the protonation of the PsbS protein. Generally, when plants are exposed to high light intensities, violaxanthin is oxidized by violaxanthin de-epoxidase (VDE). This leads to the formation of antheraxanthin, followed by zeaxanthin. Zeaxanthin creates a barrier that prevents overexcitation of the PSII reaction center. The energy from LHCII is dissipated and is not directed to the reaction center. At low light intensities, zeaxanthin epoxidation catalyzed by zeaxanthin epoxidase (ZE) occurs. The content of carotenoids, including xanthophylls participating in the xanthophyll cycle, in M and BS chloroplasts was investigated by the group of Romanowska et al.
[48]. An increase in zeaxanthin content was also observed in
Sorghum bicolor (NADP-ME plant), after exposure to the same light intensity as described above. The amount of zeaxanthin, antheraxanthin, and violaxanthin participating in the xanthophyll cycle was twice as high compared to the control
[49], which may indicate that regardless of the species, the functioning of the xanthophyll cycle is an important element of protection of the photosynthetic apparatus and dissipation of excess energy.
The participation of the LHCII antenna in energy quenching was confirmed in the mesophyll chloroplasts of maize (NADP-ME), where after the high intensity of far-red light, the LHCII were dephosphorylated, detached from the PSI in the stroma lamellae, moved to the grana, and either bound to PSII or formed aggregates which in consequence, lead to induction of the qE parameter
[46]. In M chloroplasts, light is not a factor that limits the production of ATP and NADPH, so the excess light energy is dissipated as heat.