
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angela Martins | + 2152 word(s) | 2152 | 2022-02-15 09:42:13 | | | |
| 2 | Lindsay Dong | Meta information modification | 2152 | 2022-03-23 03:00:38 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2152 | 2022-03-25 04:54:11 | | |
Video Upload Options
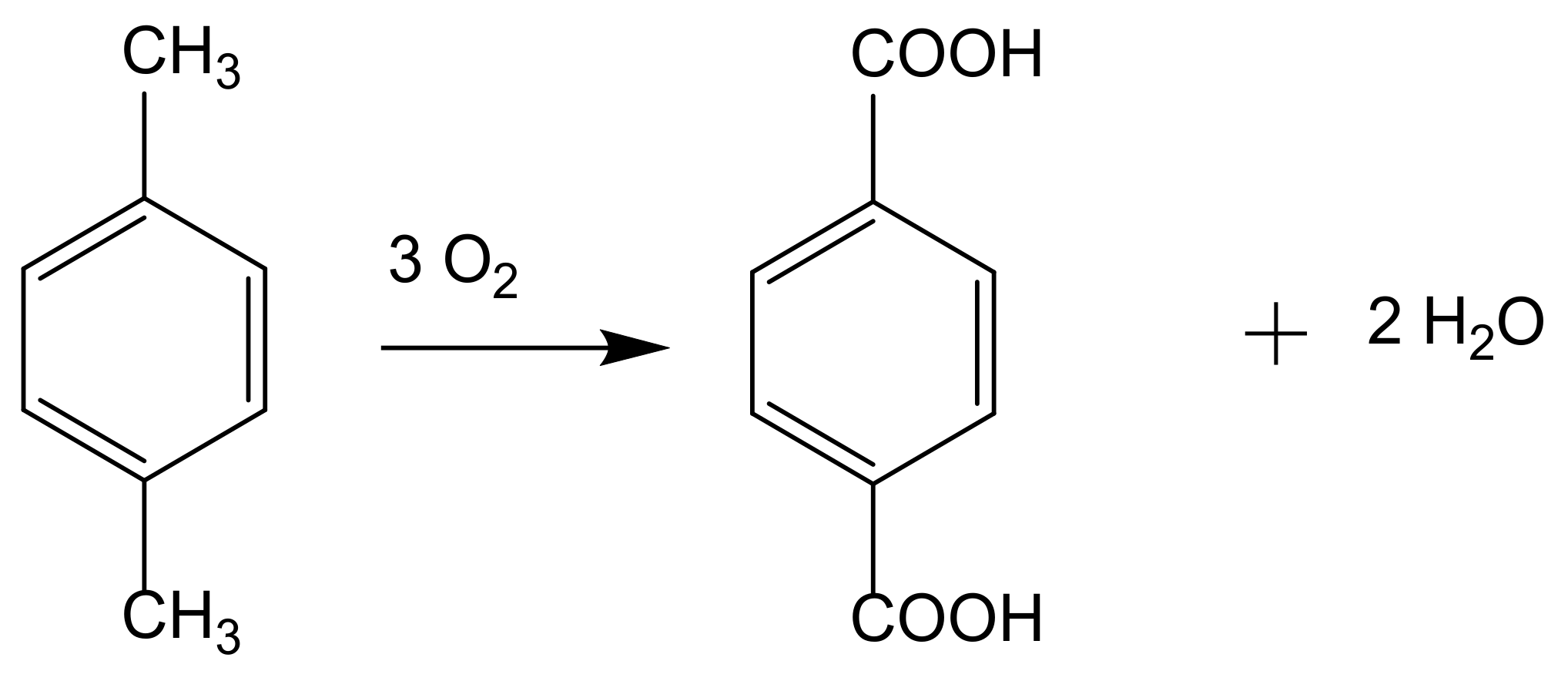
Catalytic oxidation is a key technology for the conversion of petroleum-based feedstocks into useful chemicals (e.g., adipic acid, caprolactam, glycols, acrylates, and vinyl acetate) since this chemical transformation is always involved in synthesis processes. Zeolites are microporous, crystalline aluminosilicate materials known since 1756 when the stilbite structure was identified by the Swedish mineralogist Crönstedt. Zeolites and other related porous materials can be supports for organometallic or metallic active species. These materials are the most studied supports due to their combined properties of mechanical and thermal stability that allows it an easy regeneration and recycling.
1. Industrial Hydrocarbon Oxidation Reactions
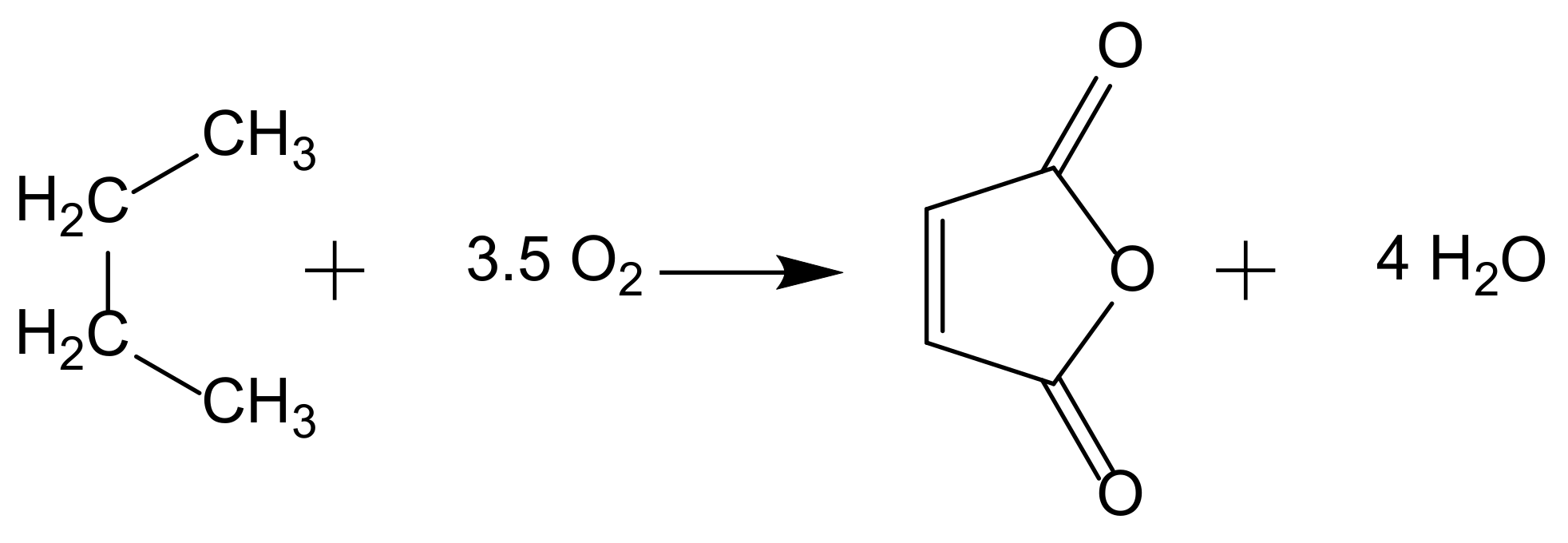
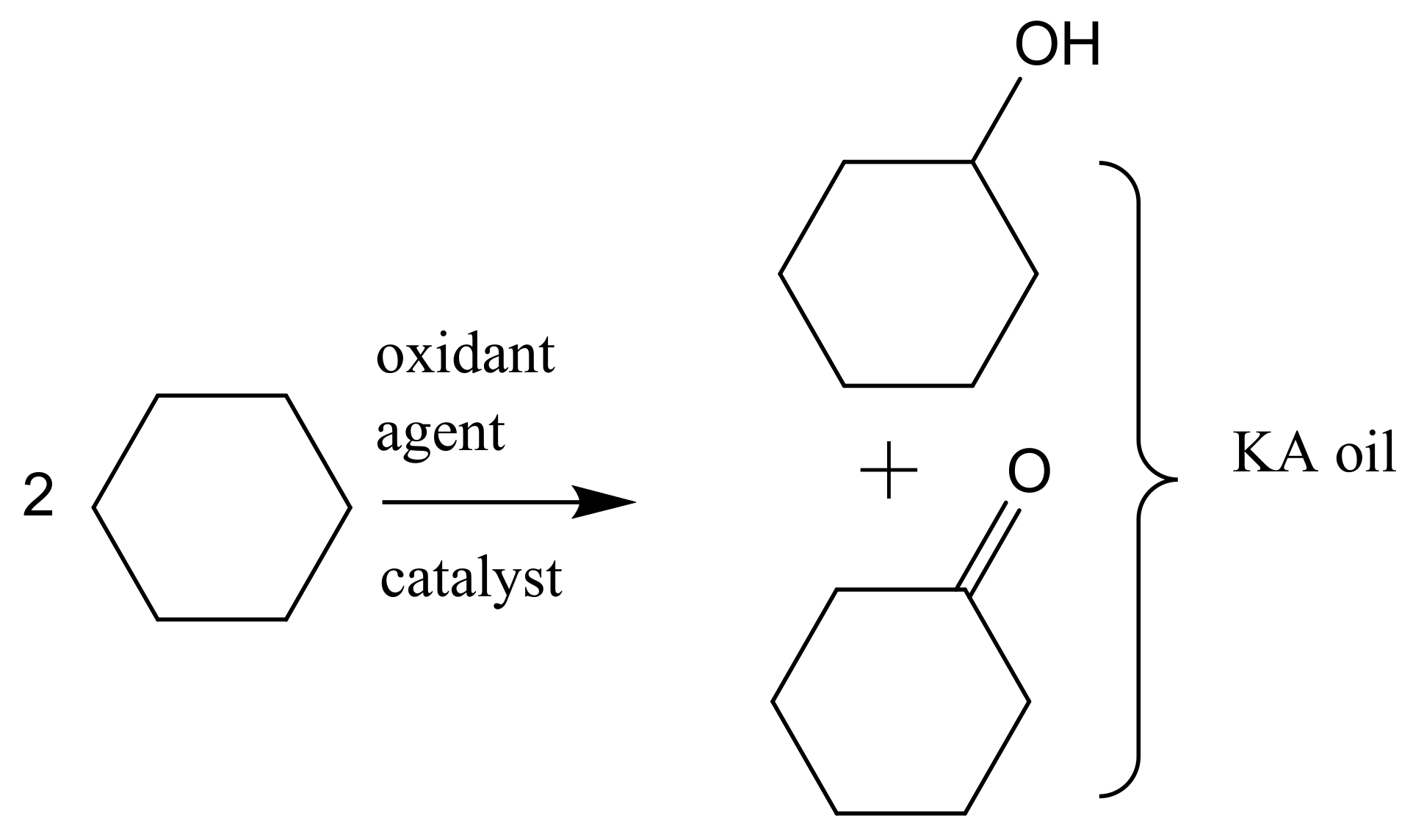
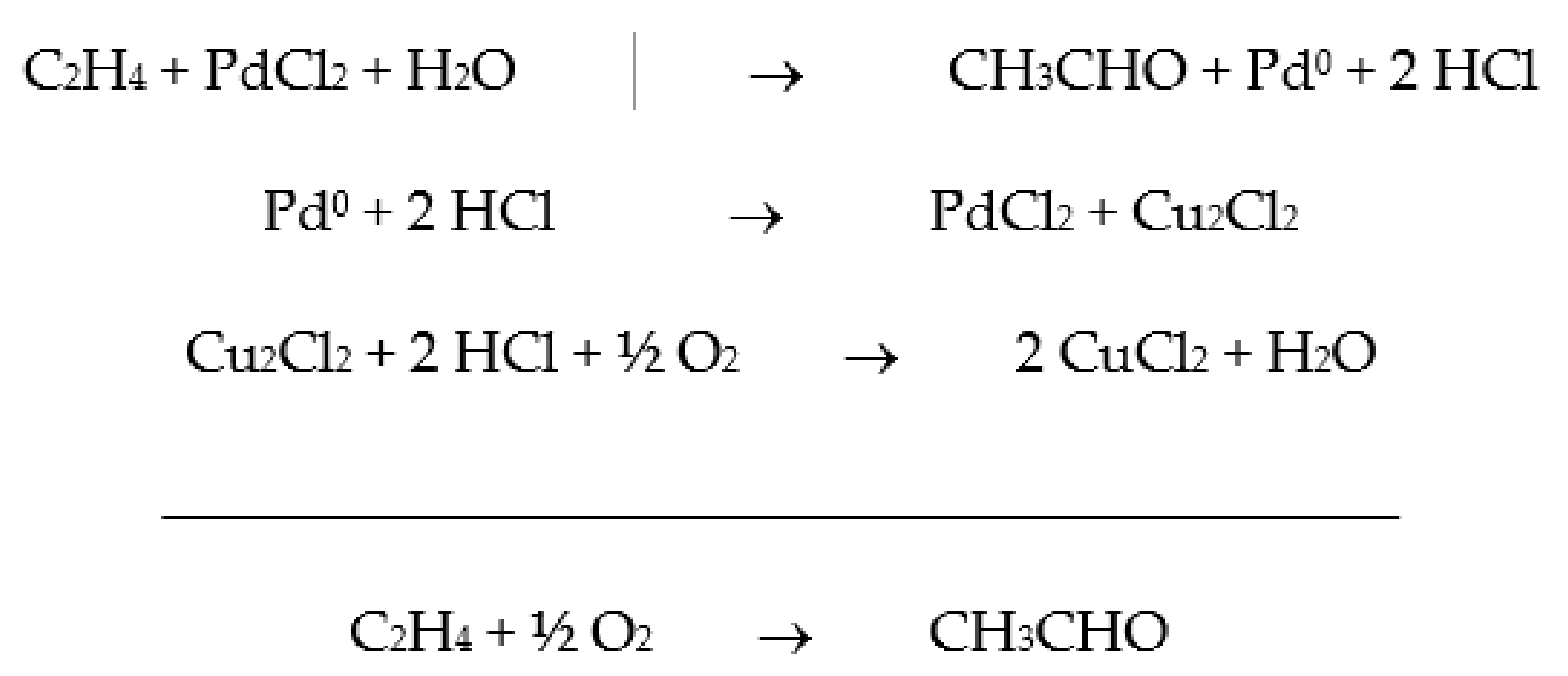
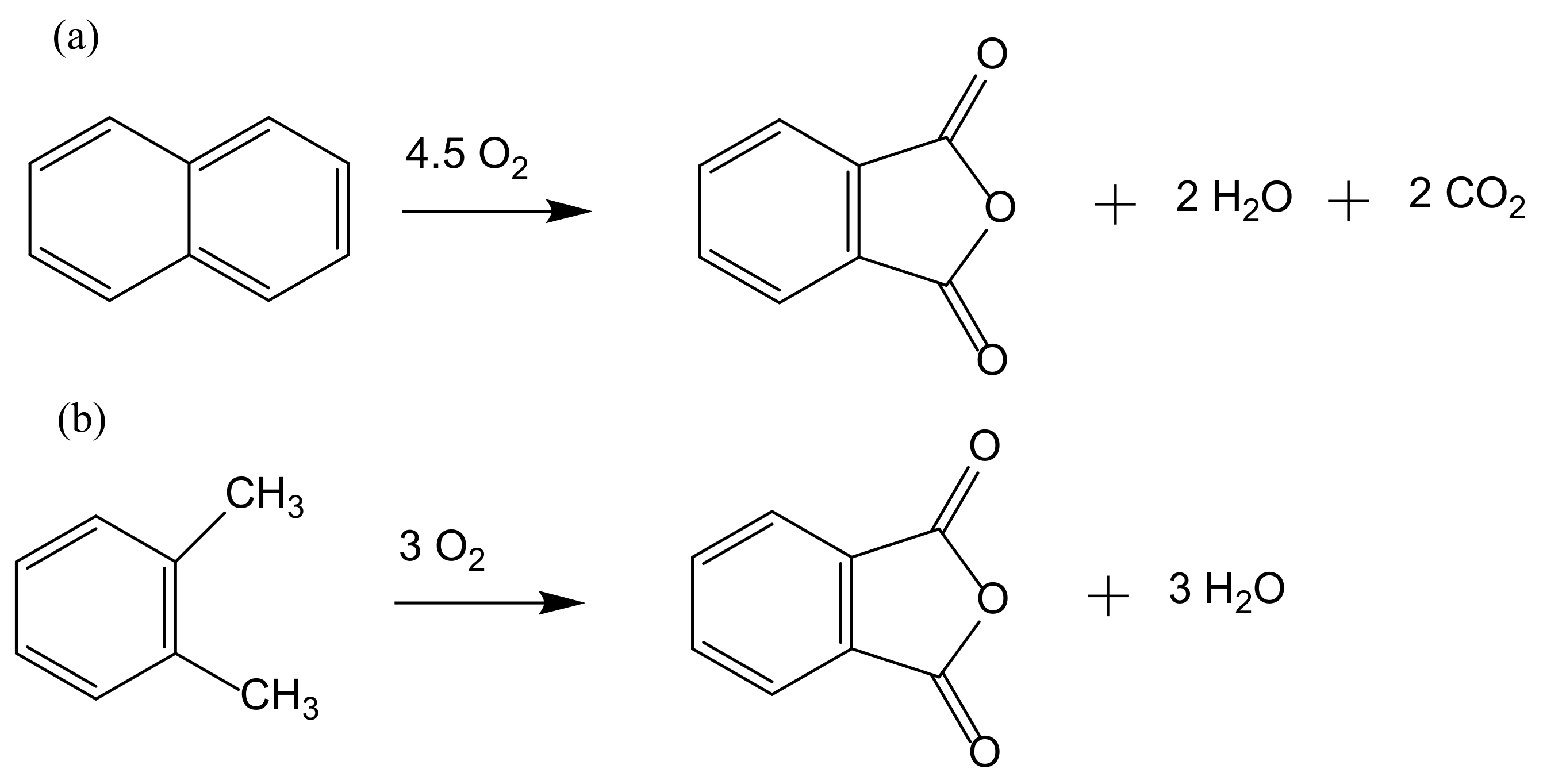
2. From Homogeneous to Heterogenized Catalysts
The development of sustainable methods for the catalytic oxidation reactions of hydrocarbons-alkanes, alkenes, and aromatics is an important scientific challenge with significant technological potential. As mentioned previously, these reactions usually occur in the presence of traditional homogeneous catalysts, such as transition and neat metals or their salts, as well as mineral acids and complexes, due to their high activity and selectivity to the desired products. However, the intensive use of these catalysts is rather controversial due to the difficult separation and recovery of the catalyst from the reaction media. The immobilization of catalytic active species in solid supports is a possible strategy to overcome some of the disadvantages of homogeneous processes. Heterogenized catalysts are easily recovered from the reaction media, without expensive separation processes and large amounts of solvents involved, with the additional advantage of allowing the reuse of the catalyst in several cycles. These are, in fact, the main objectives that one expects to achieve through the immobilization of homogeneous catalysts, but some additional benefits may also be obtained, namely when porous supports are considered. In this case, the confinement effects may enhance the interaction of the substrate with the catalyst. However, the porosity of the support may also impose some diffusional constrains that, especially when voluminous subtracts are considered, can result in an extensive loss of activity. In the case of complexes, the immobilization on solid supports has another additional benefit since it prevents dimerization phenomena that are some of the most common causes of homogeneous catalysts deactivation.
3. Zeolites and Related Materials as Support for the Heterogenization of the Catalysts
3.1. Hierarchical Zeolites
3.1.1. Bottom-Up Strategies
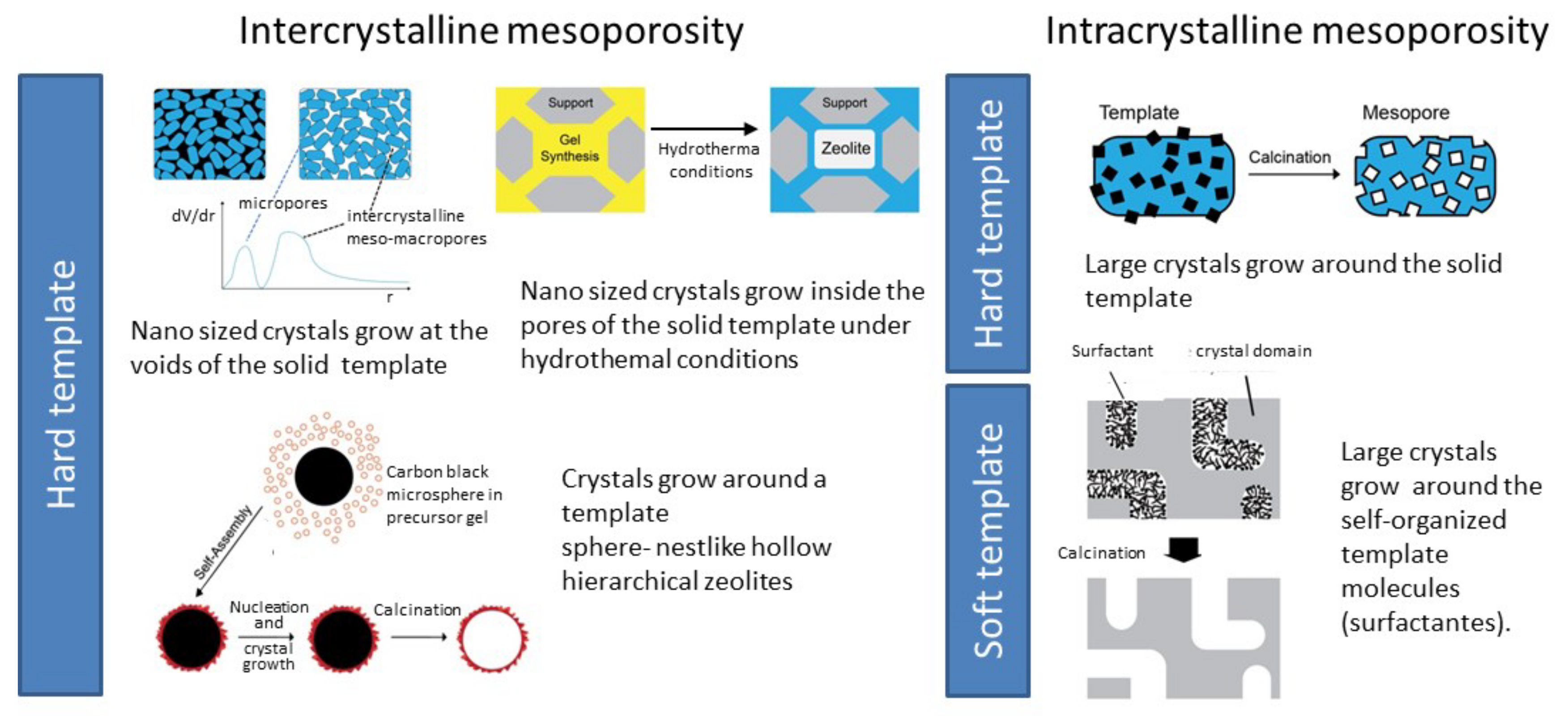
3.1.2. Top-Down Strategies
3.2. Mesoporous Silicas and Composite Hierarchical Materials
References
- Rios, J.; Lebeau, J.; Yang, T.; Li, S.; Lynch, M.D. A critical review on the progress and challenges to a more sustainable, cost competitive synthesis of adipic acid. Green Chem. 2021, 23, 3172–3190.
- Clerici, M.G.; Ricci, M.S.G. Formation of C-O bonds by oxidation. In Metal-Catalysis in Industrial Organic Processes; Gian Paolo Chiusoli, P.M.M., Ed.; Royal Society of Chemistry: London, UK, 2006; pp. 23–78.
- Martins, L.M.D.R.S. Catalytic oxidation of alkanes to high-added value products: The role of C-Scorpionate metal complexes. In Alkanes, Properties, Production and Applications; Martins, L.M.D.R.S., Ed.; Nova Science Publishers: New York, NY, USA, 2019; pp. 69–92.
- Ullmann’s Encyclopedia of Industrial Chemistry; Wiley, VCH: Weinheim, Germany, 2002.
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316.
- Ribeiro, F.R.; Guisnet, M. Les Zeolithes: Un Nanomonde au Service de la Catalyse; EDP Science: Les Ullis, France, 2006.
- Chen, N.Y.; Garwood, W.E.; Dwyer, F.G. Shape Selective Catalysis in Industrial Applications, 2nd ed.; Dekker, M., Ed.; CRC Press: New York, NY, USA, 1996.
- Schwieger, W.; Machoke, A.G.; Weissenberger, T.; Inayat, A.; Selvam, T.; Klumpp, M.; Inayat, A. Hierarchy concepts: Classification and preparation strategies for zeolite containing materials with hierarchical porosity. Chem. Soc. Rev. 2016, 45, 3353–3376.
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843.
- Trong On, D.; Desplantier-Giscard, D.; Danumah, C.; Kaliaguine, S. Perspectives in catalytic applications of mesostructured materials. Appl. Catal. A Gen. 2001, 222, 299–357.
- Mandal, S.; Sinhamahapatra, A.; Rakesh, B.; Kumar, R.; Panda, A.; Chowdhury, B. Synthesis, characterization of Ga-TUD-1 catalyst and its activity towards styrene epoxidation reaction. Catal. Commun. 2011, 12, 734–738.
- Meoto, S.; Kent, N.; Nigra, M.M.; Coppens, M.O. Effect of stirring rate on the morphology of FDU-12 mesoporous silica particles. Microporous Mesoporous Mater. 2017, 249, 61–66.
- Kishor, R.; Ghoshal, A.K. Understanding the hydrothermal, thermal, mechanical and hydrolytic stability of mesoporous KIT-6: A comprehensive study. Microporous Mesoporous Mater. 2017, 242, 127–135.
- Patcas, F.C. The methanol-to-olefins conversion over zeolite-coated ceramic foams. J. Catal. 2005, 231, 194–200.
- Mitra, B.; Kunzru, D. Washcoating of different zeolites on cordierite monoliths. J. Am. Ceram. Soc. 2008, 91, 64–70.
- Ivanova, S.; Louis, B.; Madani, B.; Tessonnier, J.P.; Ledoux, M.J.; Pham-Huu, C. ZSM-5 coatings on β-SiC monoliths: Possible new structured catalyst for the methanol-to-olefins process. J. Phys. Chem. C 2007, 111, 4368–4374.
- Louis, B.; Ocampo, F.; Yun, H.S.; Tessonnier, J.P.; Pereira, M.M. Hierarchical pore ZSM-5 zeolite structures: From micro- to macro-engineering of structured catalysts. Chem. Eng. J. 2010, 161, 397–402.
- Barg, S.; Soltmann, C.; Schwab, A.; Koch, D.; Schwieger, W.; Grathwohl, G. Novel open cell aluminum foams and their use as reactive support for zeolite crystallization. J. Porous Mater. 2011, 18, 89–98.
- Qian, X.; Du, J.; Li, B.; Si, M.; Yang, Y.; Hu, Y.; Niu, G.; Zhang, Y.; Xu, H.; Tu, B.; et al. Controllable fabrication of uniform core-shell structured composites. Chem. Sci. 2011, 2, 2006–2016.
- Qian, X.; Che, R.; Asiri, A.M. Exploring Meso-/Microporous Composite Molecular Sieves with Core-Shell Exploring Meso-/Microporous Composite Molecular Sieves with Core—Shell. Chem. Eur. J. 2012, 18, 931–939.
- Lv, Y.; Qian, X.; Tu, B.; Zhao, D. Generalized synthesis of core-shell structured mesoporous silica composites. Catal. Today 2013, 204, 2–7.
- Xu, L.; Peng, H.G.; Zhang, K.; Wu, H.; Chen, L.; Liu, Y.; Wu, P. Core-shell-structured titanosilicate as a robust catalyst for cyclohexanone ammoximation. ACS Catal. 2013, 3, 103–110.




