Catalytic oxidation is a key technology for the conversion of petroleum-based feedstocks into useful chemicals (e.g., adipic acid, caprolactam, glycols, acrylates, and vinyl acetate) since this chemical transformation is always involved in synthesis processes. Zeolites are microporous, crystalline aluminosilicate materials known since 1756 when the stilbite structure was identified by the Swedish mineralogist Crönstedt. Zeolites and other related porous materials can be supports for organometallic or metallic active species. These materials are the most studied supports due to their combined properties of mechanical and thermal stability that allows it an easy regeneration and recycling.
- hydrocarbon oxidation reactions
- zeolites
- hierarchical zeolites
- immobilized catalyst
1. Industrial Hydrocarbon Oxidation Reactions
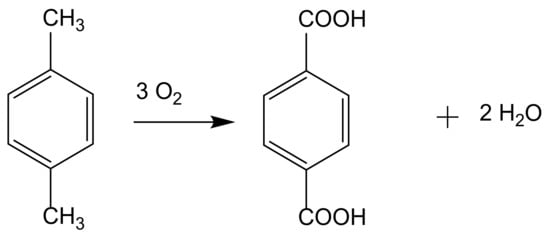
2. From Homogeneous to Heterogenized Catalysts
The development of sustainable methods for the catalytic oxidation reactions of hydrocarbons-alkanes, alkenes, and aromatics is an important scientific challenge with significant technological potential. As mentioned previously, these reactions usually occur in the presence of traditional homogeneous catalysts, such as transition and neat metals or their salts, as well as mineral acids and complexes, due to their high activity and selectivity to the desired products. However, the intensive use of these catalysts is rather controversial due to the difficult separation and recovery of the catalyst from the reaction media. The immobilization of catalytic active species in solid supports is a possible strategy to overcome some of the disadvantages of homogeneous processes. Heterogenized catalysts are easily recovered from the reaction media, without expensive separation processes and large amounts of solvents involved, with the additional advantage of allowing the reuse of the catalyst in several cycles. These are, in fact, the main objectives that one expects to achieve through the immobilization of homogeneous catalysts, but some additional benefits may also be obtained, namely when porous supports are considered. In this case, the confinement effects may enhance the interaction of the substrate with the catalyst. However, the porosity of the support may also impose some diffusional constrains that, especially when voluminous subtracts are considered, can result in an extensive loss of activity. In the case of complexes, the immobilization on solid supports has another additional benefit since it prevents dimerization phenomena that are some of the most common causes of homogeneous catalysts deactivation.
3. Zeolites and Related Materials as Support for the Heterogenization of the Catalysts
3.1. Hierarchical Zeolites
3.1.1. Bottom-Up Strategies
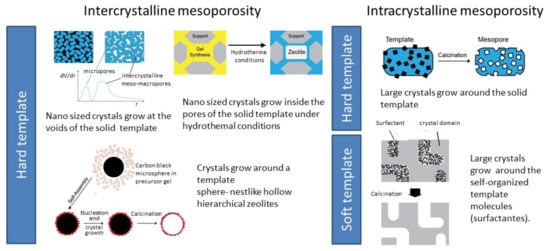
3.1.2. Top-Down Strategies
3.2. Mesoporous Silicas and Composite Hierarchical Materials
This entry is adapted from the peer-reviewed paper 10.3390/catal12020154
