
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Neelu Batra | + 2629 word(s) | 2629 | 2020-09-09 08:45:59 | | | |
| 2 | Cristabelle De Souza | + 10 word(s) | 2639 | 2020-09-13 21:55:28 | | | | |
| 3 | Camila Xu | -751 word(s) | 1888 | 2020-10-26 10:20:59 | | |
Video Upload Options
Heme oxygenase (HMOX1) is a key enzyme that catalyzes the rate-limiting first step in the heme degradation process, generating carbon monoxide, ferrous, and biliverdin, and therefore HMOX1 has a cytoprotective role as excess free heme has been shown to induce apoptosis. HMOX1 is expressed at high levels in the lungs and has been shown to mediate the anti-inflammatory effect of interleukin-10 (IL-10) in mice. Given these functions of HMOX1, it has been implicated in a variety of pathological states, including myocardial infarction, diabetes, chronic obstructive pulmonary disease (COPD). The upregulation of HMOX1 has been shown to have a protective role against the oxidative stress produced upon HIV, DENV, HCV, and IAV infections.
1. Role of HMOX1 in Thrombosis, Fibrinolysis, and Sepsis
As aggressive inflammatory responses in the respiratory tract are strongly implicated in disease severity upon SARS-CoV-2 infection, further exploration of HMOX1 function during virus infection will help to elucidate mechanisms of viral pathogenesis and potential treatments.
High-throughput genomic analyses of patients suffering from SARS-CoV-2 has provided growing evidence to suggest a sub-group of the affected population suffering from an inflammation-mediated cytokine storm. The cytokine storm, or cytokine release syndrome (CRS), is a systemic inflammatory response characterized by the innate immune system releasing a cascade of cytokines such as interferons, interleukins and chemokines, among others, thus overwhelming the host immune response leading to death [5,23,24,72]. This massive cytokine boost promotes high levels of interleukin-6 (IL-6) production, increased levels of clotting factors, and fibrinogen leading to significantly higher rates of thrombosis in COVID-19 patients [25].
Since the SARS-CoV-2 S protein binds to the human angiotensin-converting enzyme (ACE-2), many studies are currently focusing on identifying inhibitors of this interaction as potential therapeutics [8]. However, recent studies based on protein-protein interactions have demonstrated alternative pathways that could prove promising targets for therapeutic intervention or drug repurposing [26]. The SARS-CoV-2 open reading frame 3 a (ORF3a) protein was found to bind to human HMOX-1 protein with high confidence using affinity tag purification coupled to mass spectrometry (AP-MS) in an unbiased search in human cells [26]. Given the central role of inflammation in severe COVID-19 cases, this binding interaction is promising because HMOX-1 activity reduces inflammation and tissue damage [27] via the NLRP3 pathway [73,74]. This is of interest because the ORF3a in SARS-CoV-2 has been shown to directly activate the NLRP3 inflammasome pathway [28,75].
Furthermore, a number of studies that have examined the cytokine profiles of severe COVID-19 cases suggested overstimulation of macrophages and monocytes is associated with reduced T-cell abundance (lymphopenia) [29], although the exact mechanism that causes this dysregulation has yet to be identified. Importantly, macrophage differentiation into sub-classes termed M1 and M2 is mediated by the activity of HMOX-1 [30]. Surprisingly, M2 macrophages, which are considered an anti-inflammatory phenotype that inhibit T-cell activation, are upregulated in severe COVID-19 cases despite the high levels of inflammation present [29], and M2 macrophage dysfunction is associated with severe COVID-19 inflammation [26].
One simple explanation is that the anti-inflammatory activity of HMOX1 in M2 macrophages is directly inhibited by ORF3a binding. Alternatively, ORF3a binding to HMOX1 may allow viral particles to preferentially target M2 macrophages, and this could lead to several different outcomes. The ORF3a is an ion channel [76] that has a key role in viral particle release [77] and mediates both apoptotic [78] and necrotic [79] cell death. Thus, HMOX1 binding could inhibit the cytotoxic activity of the ORF3a protein in M2 macrophages, whereas in the pro-inflammatory M1 macrophages, with low levels of HMOX1, unbound ORF3a is activated and induces cell death, favoring the survival of M2 macrophages, which suppresses T-cell activation and thus allows the virus to evade T cell-mediated cell death. M2 macrophages could then act as a reservoir for viral particle production, driving further tissue destruction.
Interestingly, previous studies demonstrated that stimulation of HMOX1 production inhibits the platelet-dependent thrombus formation [80]. Likewise, increased HMOX1 expression in response to oxidative stress may represent an adaptive response mechanism to down-regulate platelet activation under prothrombotic conditions [80]. Another study showed direct evidence for a protective role of HMOX1 against thrombosis and reactive oxygen agents during vascular damage and inflammation. The induction of HMOX1 was shown to be of potential benefit in the prevention of thrombosis associated with inflammation and vascular oxidant stress [81]. Additionally, other groups have demonstrated that the products of HMOX1 possess antithrombotic properties, and impairment of HMOX1 activity might contribute to thrombus formation [82]. HMOX1 is the rate-limiting enzyme of heme degradation, which leads to the cleavage of the heme ring at the alpha methene bridge to form carbon monoxide, ionic iron, and biliverdin, which is further converted to bilirubin immediately by another enzyme, biliverdin reductase. We can see that with the pro-oxidant heme conversion to antioxidant bilirubin, HMOX-1 acts as a major antioxidant [83]. Studies showed that systemic induction of HMOX1 and bilirubin delays in vivo microvascular thrombus formation, most likely caused by a reduction in endothelial P-selectin [84]. Carbon monoxide is also shown to exert anticoagulant effects by influencing platelet aggregation [85], fibrinolysis [86] and has a role in maintaining the integrity of the vessel wall [87,88]. The fact that SARS-CoV-2 causes thrombosis, which in turn leads to patient death and the antithrombotic effects of HMOX1, clearly supports the idea that induction and upregulation/induction of HMOX1 might help to decrease thrombosis and reduce the severity of SARS-CoV-2 infection.
Furthermore, studies reported that sepsis observed in some critically ill SARS-CoV-2 patients could be resulting from an overactive immune system. Sepsis is a leading cause of death worldwide and leads to a hyper-inflammatory response, resulting in multi-organ failure. Briefly sepsis leads to red blood cell lysis that releases hemoglobin. Oxidation of hemoglobin releases free heme into the circulatory system. Proinflammatory mediators are induced by free heme, which can lead to tissue injury or cell death. HMOX1 is the enzyme responsible for heme scavenging in sepsis. Importantly, during sepsis the cytoprotective enzyme HMOX1 is upregulated and thus helps in combating sepsis-induced tissue injury. Additionally, HMOX1 plays an important role in protection from polymicrobial sepsis [89]. The HMOX1 byproduct, carbon monoxide increases phagocytic activity, thereby enhancing bacterial clearance [89,90]. Moreover, the HMOX1 product biliverdin leads to an increased expression of the anti-inflammatory mediator IL-10 and reduces expression of proinflammatory mediators such as IL-6 and MCP-1 [91]. An in vivo study report demonstrated that hemin, which is an inducer of HMOX1, decreases IL1b and IL-18 secretion, and protects from sepsis-induced acute lung injury by inhibiting the excessive inflammatory response [92]. An additional study demonstrated in vivo that HMOX1/CO plays a critical role in inhibiting LPS-mediated sepsis and pro-inflammatory cytokine production [93]. Hepatic injury caused by sepsis was also protected in mouse models via HMOX1-induced autophagy [94]. In a nutshell we can say that HMOX1 plays a protective role against polymicrobial sepsis, acute inflammation response and thrombosis that are seen in critical SARS-CoV-2 patients. Therefore, agents upregulating the HMOX1 pathway could prove to be potential therapeutic or preventive agents for SARS-CoV-2.
2. Natural Antiviral Agents that Upregulate the HMOX1 Pathway
In addition to synthetic molecules that are potential treatment strategies for SARS-CoV-2 viral infection, it is important to note some compounds/extracts obtained from natural plants that could show promise for therapy. One such natural plant is the neem plant (Azardirachta indica), known for its anti-inflammatory, antioxidant, antimalarial, antiarthritic, antipyretic, hypoglycemic, antigastric ulcer, antifungal, antibacterial, and antitumour activities or immune-stimulating properties [107,108,109,110,111,112]. There are currently more than 140 biologically active compounds including Nimbolide that has been shown to be a unique druggable modality especially in cancer pathogenicity [113]. Nimbolide is a limonoid tetranortriterpenoid with an α,β-unsaturated ketone system and a δ-lactone ring isolated from Azadirachta indica. A study showed that aqueous neem bark extract (NBE) preparation from remarkably blocked herpes simplex virus 1 (HSV-1) entry into natural target cells at concentrations ranging from 50–100 µg/mL. [114]. Many reports determined that neem extracts significantly inhibited various viruses such as coxackie B group virus, poliovirus, dengue virus and HIV at early steps of viral genome replication. In vitro and in vivo studies have described the inhibitory potential of crude aqueous extract of neem leaves and pure neem compound (Azadirachtin) on the replication of dengue virus type-2 [115]. Azadirachtin is a triterpenoid found in need tree seeds. Studies have reported the antiviral properties of Azadirachta indica polysaccharides for poliovirus in vitro [116], by mechanistically inhibiting initial stages of viral replication. The neem extract was also found to act as a viricidal agent against the coxsackie virus B-4 [117]. Water-extracted polysaccharides from neem leaves exerted anti-bovine herpesvirus type 1 (BoHV-1) activity and a fractionated acetone-water neem extract, IRAB is being marketed as a drug against HIV, malaria, and cancer in Nigeria under the trade name IRACAP [118]. Additional groups have shown the antiviral activity of neem seed kernel extracts in vitro against duck plague virus (Table 2) [119].
Table 2. Antiviral properties of neem.
| Neem Plant Part | Virus Type | Reference |
|---|---|---|
| Aqueous extract preparation from the barks of neem (NBE) | Herpes Simplex Virus 1 (HSV-1) | [114] |
| Crude aqueous extract of neem leaves and pure neem compound (Azadirachtin) | Dengue virus type 2 | [115] |
| Neem’s polysaccharides extracted from leaves | Poliovirus | [116] |
| Neem leaf extract | Coxsackie virus B-4 | [117] |
| Water-extracted polysaccharides from neem leaves | Anti-bovine herpesvirus Type 1 | [118] |
| Neem seed kernel extracts | Duck plague virus | [119] |
| Fractionated neem-leaf extract | Human Immunodeficiency Virus 1 (HIV-1) | [120] |
3. Upregulation of Protein and mRNA Levels of HMOX1 Using Natural Compounds and Clinically Available Therapeutics
While the neem plant may not be the only natural compound that has therapeutic potential for its anti-viral properties, previous reports showed that the neem leaf extract upregulates the HMOX1 protein and mRNA expression [121]. One such study reported a significant increase in the HMOX1 protein level in C4-2B and PC-3M-luc2 prostate cancer cells after 24 and 48 h of treatment with the EENL (ethanolic extract of neem leaves) [108]. These results are consistent with the increase in the mRNA expression levels of the HMOX1 gene after EENL treatment. These results were validated by another study showing significant overexpression of HMOX1 RNA expression in HUVEC cells post treatment with 20.0 and 40.0 μg/mL of EENL for 24 h [108]. Yet another study evaluated compounds present in the leaves of the neem tree (Azadirachta Indica) as potential inhibitors for COVID-19 main protease (Mpro) (PDB code: 6LU7). The main protease (Mpro, also called 3CLpro) is an attractive drug target among coronaviruses because of its essential role in processing the polyproteins that are translated from the viral RNA. They used blind molecular docking using PyRx and Auto Vina software to compare the binding energies obtained from the docking of 6LU7 with meliacinanhydride, nimocinol, isomeldenin, nimbolide, zafaral, nimbandiol, nimbin, nimbinene, desacetylnimbin and hydroxychloroquine and remdesivir as positive controls. They found that meliacinanhydride (Ki = 33.36 pM) and the compounds from neem leaves had significantly high binding energy to 6LU7, which could point to a potential clinical drug option against COVID-19. Additionally, neem leaves also contain other immunity boosting compounds such as quercetin, zinc, vitamin A, B1, B2, B6, C, E [122,123].
To our understanding, this could be a great direction for further investigation of natural plants such as neem as potential therapeutic options for SARS-CoV-2 (Figure 2). Other agents that can upregulate HMOX1 should be explored and drugs that can be repurposed to upregulate or modulate the HMOX1 pathway should be urgently evaluated.
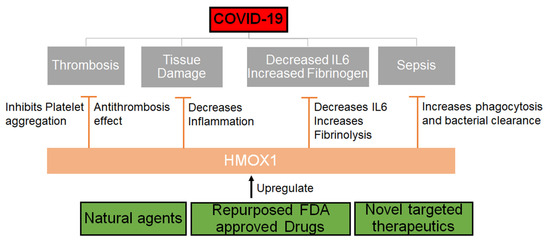
Figure 2. Mechanism through which HMOX1 can be regulated. HMOX1 that can be upregulated by either natural compounds or synthetic molecules may inhibit the effects of SARS-CoV-2 by decreasing inflammation, IL6 levels and increasing phagocytosis, fibrinolysis and thereby inhibiting thrombosis and sepsis.




